
Tracks 1-11 |
| Track 1 |
Introduction |
| Track 2 |
Adjuvant chemotherapy for
Stage II colon cancer |
| Track 3 |
Development and validation of a prognostic assay in colon cancer |
| Track 4 |
Perspectives on ECOG-E5202 adjuvant trial design |
| Track 5 |
NSABP-C-08: FOLFOX with or without bevacizumab in the adjuvant setting |
| Track 6 |
Future adjuvant NSABP colon trial design |
|
| Track 7 |
Emerging clinical research on panitumumab |
| Track 8 |
NSABP-C-09: CAPOX with or without hepatic arterial infusion of FUDR following hepatic resection |
| Track 9 |
NSABP-C-10: FOLFOX plus bevacizumab for patients with a synchronous primary lesion and metastatic disease |
| Track 10 |
Perspectives on NSABP-R-04 trial design |
| Track 11 |
Adjuvant chemotherapy for
rectal cancer |
|
|
Select Excerpts from the Interview and the CME Symposium
Tracks 2-3
 DR LOVE:
DR LOVE: Can you discuss current controversies in the treatment of patients with Stage II colon cancer?
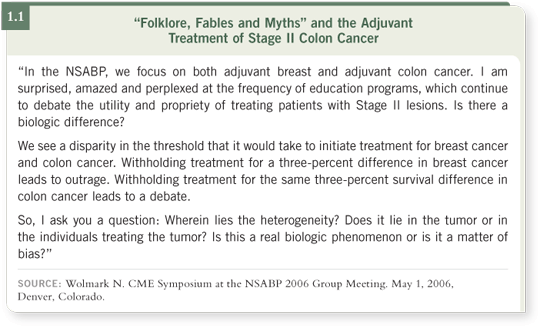
 DR WOLMARK: When all is said and done, the Stage II controversy entails no basic disagreement. It’s a matter of degree and of perspective (1.1). We don’t advocate that all patients with Stage II disease be treated — we advocate that patients with Stage II disease be included in the discussion.
DR WOLMARK: When all is said and done, the Stage II controversy entails no basic disagreement. It’s a matter of degree and of perspective (1.1). We don’t advocate that all patients with Stage II disease be treated — we advocate that patients with Stage II disease be included in the discussion.
 DR LOVE: Similarly in breast cancer, not all node-negative patients receive chemotherapy.
DR LOVE: Similarly in breast cancer, not all node-negative patients receive chemotherapy.
 DR WOLMARK: Precisely. Just don’t be authoritarian. Enter the patients into clinical trials, talk to them about the risks and benefits and make the decisions.
DR WOLMARK: Precisely. Just don’t be authoritarian. Enter the patients into clinical trials, talk to them about the risks and benefits and make the decisions.
 DR LOVE: I think the heterogeneity that I’ve seen is in the clinical investigator
communities. Some are really on board with the relative risk concept in colon cancer, but others are not.
DR LOVE: I think the heterogeneity that I’ve seen is in the clinical investigator
communities. Some are really on board with the relative risk concept in colon cancer, but others are not.
 DR WOLMARK: It’s a completely different mindset, and I believe a lot of it has
been due to the higher level of advocacy in other cancers. In breast cancer, if
you don’t treat for a three percent survival difference, you’ll have outrage.
DR WOLMARK: It’s a completely different mindset, and I believe a lot of it has
been due to the higher level of advocacy in other cancers. In breast cancer, if
you don’t treat for a three percent survival difference, you’ll have outrage.
 DR LOVE: Can you talk about your take on the new data by the NSABP and
Genomic Health that identified genes associated with prognosis in patients
with colon cancer?
DR LOVE: Can you talk about your take on the new data by the NSABP and
Genomic Health that identified genes associated with prognosis in patients
with colon cancer?
 DR WOLMARK: Out of approximately 700 candidate genes, 140 genes, more
or less, had independent prognostic significance in univariate analysis. If one
performed multivariate analysis, controlling for the number of nodes and other
variables, they remained significant.
DR WOLMARK: Out of approximately 700 candidate genes, 140 genes, more
or less, had independent prognostic significance in univariate analysis. If one
performed multivariate analysis, controlling for the number of nodes and other
variables, they remained significant.
I believe that’s good news. I certainly consider it feasible to work out an
Oncotype DX™-like assay for colon cancer. The challenge will be validating it
in a pristine subset, for which the tumor tissue bank is intact. That’s something
that can and will be done.
Track 5
 DR LOVE:
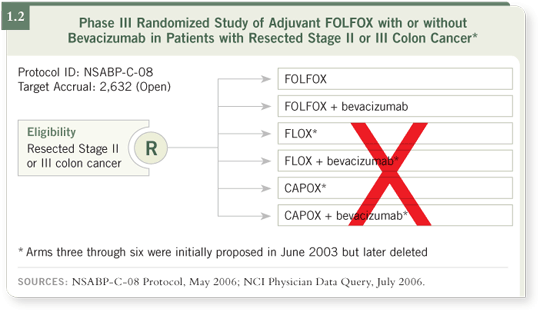
DR LOVE: Let’s talk about some of the NSABP trials that are going on
right now, starting with C-08 (1.2), evaluating the modified FOLFOX-6
regimen with or without bevacizumab. Where are we in terms of accrual,
and how are physicians responding to that study?
 DR WOLMARK: It’s accruing very well. It started in September of 2004, and
we’ve passed the 2,000 mark. The required sample size is 2,632. So we’ve had
as good a response from the membership as we can possibly get. People are
enthusiastic about using bevacizumab or assessing bevacizumab in the adjuvant
setting.
DR WOLMARK: It’s accruing very well. It started in September of 2004, and
we’ve passed the 2,000 mark. The required sample size is 2,632. So we’ve had
as good a response from the membership as we can possibly get. People are
enthusiastic about using bevacizumab or assessing bevacizumab in the adjuvant
setting.
 DR LOVE: The adjuvant trastuzumab studies (Piccart-Gebhart 2005; Romond
2005) seem to have increased enthusiasm about the colon adjuvant bevacizumab studies because they use a similar model. Patients may be treated with a
promising therapy that they wouldn’t otherwise receive.
DR LOVE: The adjuvant trastuzumab studies (Piccart-Gebhart 2005; Romond
2005) seem to have increased enthusiasm about the colon adjuvant bevacizumab studies because they use a similar model. Patients may be treated with a
promising therapy that they wouldn’t otherwise receive.
 DR WOLMARK: I don’t disagree. What further stimulated interest is that the
results with trastuzumab were so spectacular. In some ways, it raises enormous
expectations for what bevacizumab will do in the adjuvant setting.
DR WOLMARK: I don’t disagree. What further stimulated interest is that the
results with trastuzumab were so spectacular. In some ways, it raises enormous
expectations for what bevacizumab will do in the adjuvant setting.
I certainly don’t expect it will do what trastuzumab did in breast cancer. We
had a targeted subset of women whose tumors were HER2-positive. We don’t
have the same kind of population in the adjuvant setting in C-08.
On the other hand, “hope springs eternal,” and I hope to see robust differences. The level of efficacy of trastuzumab in the adjuvant setting was a once-in-a-lifetime observation for some of us. Perhaps we’ll be lucky and see it twice.
Track 6
 DR LOVE:
DR LOVE: In terms of the issue of safety with bevacizumab — particularly as it relates to patients being treated in the adjuvant setting — what’s
your take right now on where we are regarding arterial events — in
particular, among patients with prior arterial events or older patients?
 DR WOLMARK: We’ve restricted trial entry relative to prior events and prior
myocardial infarctions. We hope to have a population that will not be at
increased risk. This trial is being monitored very carefully and, to date, we
haven’t seen anything untoward or unexpected.
DR WOLMARK: We’ve restricted trial entry relative to prior events and prior
myocardial infarctions. We hope to have a population that will not be at
increased risk. This trial is being monitored very carefully and, to date, we
haven’t seen anything untoward or unexpected.
 DR LOVE: With the idea that the accrual for the trial will be complete
amazingly soon, can you talk about the discussions that are going on about a
replacement study?
DR LOVE: With the idea that the accrual for the trial will be complete
amazingly soon, can you talk about the discussions that are going on about a
replacement study?
 DR WOLMARK: The most enthusiasm for replacing C-08 would be in studying
a double antibody regimen. Of course, the candidates would be bevacizumab
with panitumumab, or bevacizumab with cetuximab, compared to bevacizumab alone, together with chemotherapy in both arms.
DR WOLMARK: The most enthusiasm for replacing C-08 would be in studying
a double antibody regimen. Of course, the candidates would be bevacizumab
with panitumumab, or bevacizumab with cetuximab, compared to bevacizumab alone, together with chemotherapy in both arms.
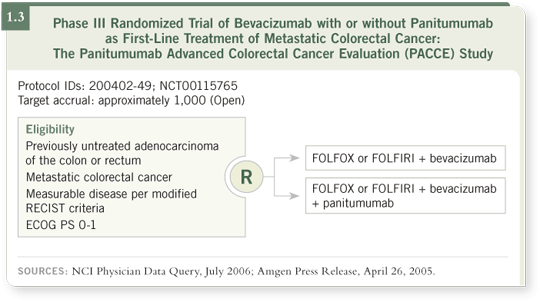
From what we have heard to date, the preference would be to use bevacizumab with panitumumab, seeking both an efficacy signal and a safety signal,
provided we have supporting data from the PACCE trial (Panitumumab
Advanced Colorectal Cancer Evaluation; [1.3]).
Track 7
 DR LOVE:
DR LOVE: Why the focus on panitumumab as opposed to cetuximab?
 DR WOLMARK: A general level of interest emerges when you have a fully
human monoclonal antibody. It acquires some “designer cachet.” It’s certainly
not based on a greater repository of data or any head-on comparisons. It’s a
matter of preference, and it’s a matter of who is an active proponent of it.
DR WOLMARK: A general level of interest emerges when you have a fully
human monoclonal antibody. It acquires some “designer cachet.” It’s certainly
not based on a greater repository of data or any head-on comparisons. It’s a
matter of preference, and it’s a matter of who is an active proponent of it.
 DR LOVE: How do you think the panitumumab-associated rash will play out
in the adjuvant setting? We’re starting to consider this in the adjuvant lung
cancer setting, with the tyrosine kinase inhibitors.
DR LOVE: How do you think the panitumumab-associated rash will play out
in the adjuvant setting? We’re starting to consider this in the adjuvant lung
cancer setting, with the tyrosine kinase inhibitors.
 DR WOLMARK: Patients, particularly in the adjuvant setting, if given an
opportunity to increase their survival, will be compliant. In the adjuvant
setting, of course, it will be temporary, which is different from what you
would have in other settings. So given the opportunity to increase cure rates,
I believe the patients will be compliant if they know that the rash will occur
and then resolve.
DR WOLMARK: Patients, particularly in the adjuvant setting, if given an
opportunity to increase their survival, will be compliant. In the adjuvant
setting, of course, it will be temporary, which is different from what you
would have in other settings. So given the opportunity to increase cure rates,
I believe the patients will be compliant if they know that the rash will occur
and then resolve.
We went through issues of total alopecia in breast cancer, and we were
told that nobody would tolerate it. Predictions were totally incorrect in the
adjuvant setting, and it was not an insurmountable problem.
Track 8
 DR LOVE:
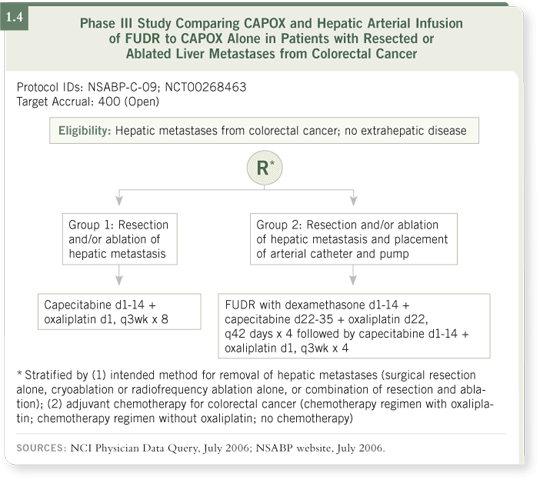
DR LOVE: What are your thoughts about the new NSABP-C-09 trial of hepatic resection or ablation followed by CAPOX chemotherapy with or without intrahepatic FUDR (1.4)?
 DR WOLMARK: The response we’ve received to date has been one that would perhaps engender cautious optimism as to the likelihood of our being able to carry out this trial. We need 400 patients, and we need those 400 patients referred by individuals who have experience in that area.
DR WOLMARK: The response we’ve received to date has been one that would perhaps engender cautious optimism as to the likelihood of our being able to carry out this trial. We need 400 patients, and we need those 400 patients referred by individuals who have experience in that area.
 DR LOVE: My sense — and we’ve seen this in our Patterns of Care studies — is that there’s a lot more sensitivity and activity among medical oncologists
looking for curative situations in metastatic disease. So hopefully it will increase the denominator you might have available.
DR LOVE: My sense — and we’ve seen this in our Patterns of Care studies — is that there’s a lot more sensitivity and activity among medical oncologists
looking for curative situations in metastatic disease. So hopefully it will increase the denominator you might have available.
 DR WOLMARK: We’ve gotten a good response from the CTSU as far as IRB approval. We see no reason to believe that this trial will not be successful at this point, but after one patient’s been randomly assigned, I can say whatever I like. We’ll revisit this issue in six to eight months, and then we’ll have a much better idea.
DR WOLMARK: We’ve gotten a good response from the CTSU as far as IRB approval. We see no reason to believe that this trial will not be successful at this point, but after one patient’s been randomly assigned, I can say whatever I like. We’ll revisit this issue in six to eight months, and then we’ll have a much better idea.
 DR LOVE: The NSABP has always attempted trials that have difficult randomizations, and this one’s not easy.
DR LOVE: The NSABP has always attempted trials that have difficult randomizations, and this one’s not easy.
 DR WOLMARK: I certainly agree with you on both those issues; we’ve attempted difficult trials, and this is one of the more difficult ones.
DR WOLMARK: I certainly agree with you on both those issues; we’ve attempted difficult trials, and this is one of the more difficult ones.
Track 9
 DR LOVE:
DR LOVE: Can you talk about the NSABP-C-10 study of FOLFOX with bevacizumab for patients with synchronous metastases?
 DR WOLMARK: The C-10 study addresses a practical question: When you have a synchronous presentation of a primary lesion with metastatic disease, the standard has been to resect the primary lesion because you want to prevent the subsequent complications of bleeding, fistula formation, erosion into other organs and so on. Is that necessary, particularly when you have promising new agents such as oxaliplatin and bevacizumab?
DR WOLMARK: The C-10 study addresses a practical question: When you have a synchronous presentation of a primary lesion with metastatic disease, the standard has been to resect the primary lesion because you want to prevent the subsequent complications of bleeding, fistula formation, erosion into other organs and so on. Is that necessary, particularly when you have promising new agents such as oxaliplatin and bevacizumab?
This is a Phase II trial with a sample size of 90 and complications of obstruction,
bleeding and fistula formation and so on as the primary endpoints. So it’s a feasibility trial. The intervention is FOLFOX with bevacizumab.
 DR LOVE: Obviously, some selection will be involved in terms of patients with critical local problems who need surgery and who probably aren’t going to be enrolled into this study. But, assuming the more typical situation, in which a patient’s primary lesion is stable, one of the issues is that if they do develop some kind of problem that requires surgery, you’re going to have bevacizumab on board, which may be an issue.
DR LOVE: Obviously, some selection will be involved in terms of patients with critical local problems who need surgery and who probably aren’t going to be enrolled into this study. But, assuming the more typical situation, in which a patient’s primary lesion is stable, one of the issues is that if they do develop some kind of problem that requires surgery, you’re going to have bevacizumab on board, which may be an issue.
 DR WOLMARK: Yes. It may be, but there’s one way to find out. And, again,
I don’t say that flippantly. If we can demonstrate that you’re going to get
response in the primary site and elsewhere and that the rate of complications
will be kept within the protocol limits, it should be a step forward.
DR WOLMARK: Yes. It may be, but there’s one way to find out. And, again,
I don’t say that flippantly. If we can demonstrate that you’re going to get
response in the primary site and elsewhere and that the rate of complications
will be kept within the protocol limits, it should be a step forward.
 DR LOVE: What do we know about the response of primary tumors to our
current systemic therapies, particularly those that include bevacizumab?
DR LOVE: What do we know about the response of primary tumors to our
current systemic therapies, particularly those that include bevacizumab?
 DR WOLMARK: I don’t know a great deal. We have the Willett data (Willett
2004), for example, for rectal tumors. I’m not sure we know a whole lot using
bevacizumab. Regarding what it does to the primary tumors in colorectal
cancer, I’m not sure there’s much out there to allow us to make reasonable
estimates.
DR WOLMARK: I don’t know a great deal. We have the Willett data (Willett
2004), for example, for rectal tumors. I’m not sure we know a whole lot using
bevacizumab. Regarding what it does to the primary tumors in colorectal
cancer, I’m not sure there’s much out there to allow us to make reasonable
estimates.
Select publications

