
The colorectal cancer plenary session at the 2003 American Society of Clinical Oncology annual meeting was a milestone in oncologic research. For the first time, a major randomized adjuvant clinical trial (MOSAIC) demonstrated a significant advantage in disease-free survival with the addition of a second systemic agent (oxaliplatin) to a fluoropyrimidine.
Shortly after Aimery de Gramont presented these fascinating results, Herb Hurwitz reviewed the findings from another study that set the stage for what has quickly become the fourth strategy in systemic cancer therapy. In a stunning confirmation of the hypotheses posed by Judah Folkman and others, the anti-VEGF monoclonal antibody bevacizumab was found to significantly prolong progression-free and overall survival when added to IFL in the first-line metastatic setting. Now, in addition to cytotoxic treatment, endocrine therapy and agents such as trastuzumab that target growth-factor receptors on and in cancer cells, we had anti-angiogenesis as part of our treatment armamentarium.
Several weeks after that ASCO meeting, Dr Norman Wolmark quipped during the NSABP group meeting in Orlando that, for the first time, more people attended the ASCO colorectal cancer presentations than the breast cancer sessions. The most discussed future clinical trial at that NSABP meeting was C-08, which started out as a three-by-two factorial design comparing FLOX to FOLFOX to CAPOX with or without bevacizumab. This landmark trial eventually morphed into its current, more focused form comparing FOLFOX with or without bevacizumab — a study design few of us would have anticipated several years earlier.
At the most recent NSABP group meeting in Denver on May 1, our education
group had the honor of conducting a CME symposium during the scientific
session to review recent important and related colorectal cancer clinical research developments since that historic 2003 ASCO meeting. The enclosed audio program includes highlights of that meeting and individual interviews with the speakers. The following issues are addressed:
1. Adjuvant systemic therapy for Stage II colon cancer
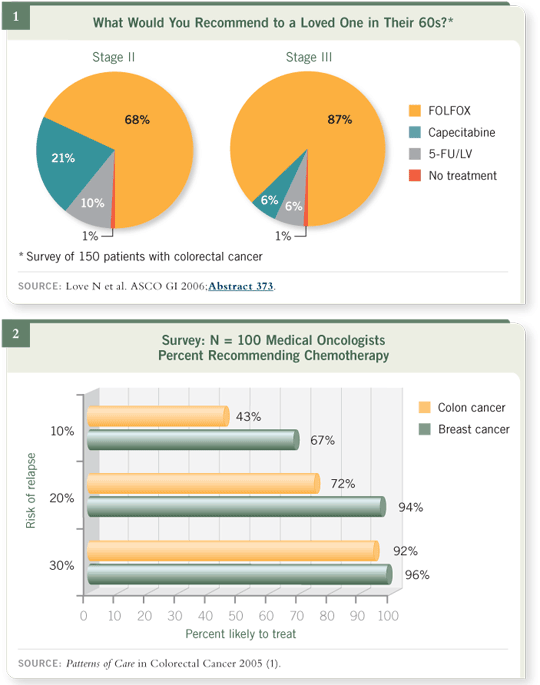
Dr Wolmark reviewed this controversy, which was fueled by a much-discussed ASCO position paper and the FDA approval of oxaliplatin, which was restricted to Stage III disease despite the fact that 40 percent of patients
in the MOSAIC trial had Stage II tumors. A related survey of 150 people
with colorectal cancer that our CME group reported at the 2006 ASCO GI
meeting demonstrated that, after listening to a 50-minute audio CD of
Dr John Marshall describing in detail the risks and benefits of various adjuvant
systemic therapies, patients preferred systemic therapies in a manner similar to
other prior surveys of breast cancer patients (Figure 1). Of great interest is a
recent related national Patterns of Care study of medical oncologists suggesting
that the recurrence bar to trigger adjuvant therapy is significantly lower in
breast cancer than in colon cancer (Figure 2).
2. What is the current optimal method to deliver oxaliplatin and a fluoropyrimidine in the adjuvant and metastatic settings?
Dr Wolmark presented results from NSABP-C-07 for the first time at the 2005 ASCO meeting, suggesting that FLOX, which uses bolus 5-FU, is an alternative to FOLFOX, which includes continuous infusion 5-FU. However, our CME group’s Patterns of Care research demonstrates that adjuvant FOLFOX is currently prescribed much more commonly than FLOX.
A related issue is whether capecitabine can be substituted for 5-FU. The three-arm AVANT study is addressing that issue. During the symposium, Dr Howard Hochster discussed the TREE studies, which have resulted in encouraging
data on the use of capecitabine/oxaliplatin combined with bevacizumab in the first-line metastatic setting.
3. What is the optimal fluoropyrimidine monotherapy in the adjuvant setting?
This issue seemed to be resolved with the 2004 ASCO presentation of the X-ACT trial, demonstrating an advantage in safety and relapse-free survival with capecitabine compared to the Mayo Clinic regimen of 5-FU/leucovorin. Our Patterns of Care work demonstrates a rapid uptake in the use of adjuvant capecitabine as monotherapy. It is interesting that in breast cancer, CALGB trial 49907 — comparing capecitabine to AC or CMF in patients older than age 65 — is limping along in accrual, whereas colorectal cancer has at least moved beyond the important question of whether orally administered chemotherapy
can be substituted for intravenous treatment.
4. What is the current research database on the safety of bevacizumab?
The NSABP-C-08 research question of chemotherapy with or without bevacizumab is clearly the dominant issue in the current adjuvant colorectal trials, including AVANT, ECOG-E5202 in patients with high-risk Stage II disease and ECOG-E5204 in patients with rectal cancer. The Hurwitz IFL/ bevacizumab trial heightened our awareness of the unusual complications of bowel perforation and hypertension associated with bevacizumab. Dr Hurwitz will update us on further work in this critical area, including the increased risk of arterial events. While the recent spectacular results from the adjuvant trastuzumab trials in breast cancer have resulted in optimism that monoclonal antibody therapy in the adjuvant setting might yield positive outcomes in colorectal cancer, to date there are minimal long-term toxicity data with bevacizumab. On this program, Herb Hurwitz updates what we do know currently.
5. What is the current and future role of anti-EGFR therapy in the adjuvant and metastatic settings?
Dr John Marshall provided an update on trials of monoclonal antibodies, cetuximab and panitumumab. A presentation at the April AACR meeting in Washington, DC of a trial in the metastatic setting comparing panitumumab with best supportive care to best supportive care with an optional crossover to panitumumab demonstrated, for the first time, a progression-free survival advantage to anti-EGFR monotherapy. Combined targeted biologic therapy is another promising treatment strategy, and Dr Hochster reviewed findings from the BOND-2 study, which combined an EGFR antagonist (cetuximab) with bevacizumab.
These recent research advances leave oncology healthcare professionals and their patients optimistic that the management of this disease has moved past the days of 5-FU, 5-FU and 5-FU into new and very exciting territory.
— Neil Love, MD
NLove@ResearchToPractice.net
Select publications

