
Tracks 1-11 |
| Track 1 |
Introduction |
| Track 2 |
EGFR inhibitors in the management of colon cancer |
| Track 3 |
Clinical trial experience with cetuximab |
| Track 4 |
Synergy between cetuximab and chemotherapy |
| Track 5 |
Similarities and differences between panitumumab and cetuximab |
| Track 6 |
Incorporating EGFR inhibitors into the treatment algorithm |
|
| Track 7 |
Management of rash associated with EGFR inhibitors |
| Track 8 |
Potential role of panitumumab
in clinical practice |
| Track 9 |
Adjuvant chemotherapy for Stage II colon cancer |
| Track 10 |
Adjuvant capecitabine for patients with Stage II disease |
| Track 11 |
Case discussion: An 84-year-old man with Stage II colon cancer |
|
|
Select Excerpts from the Interview and the CME Symposium
Track 3
 DR LOVE:
DR LOVE: Can you briefly describe what we know about cetuximab?
 DR MARSHALL: Cetuximab arrived on the scene in a very dramatic fashion. Leonard Saltz conducted the first trial that showed us the strength and power of this drug in the United States (Saltz 2001). This was a fairly simple Phase II clinical trial in which patients who were refractory to everything that we had at the time were given cetuximab in combination with irinotecan, even though patients had already experienced progression on irinotecan.
DR MARSHALL: Cetuximab arrived on the scene in a very dramatic fashion. Leonard Saltz conducted the first trial that showed us the strength and power of this drug in the United States (Saltz 2001). This was a fairly simple Phase II clinical trial in which patients who were refractory to everything that we had at the time were given cetuximab in combination with irinotecan, even though patients had already experienced progression on irinotecan.
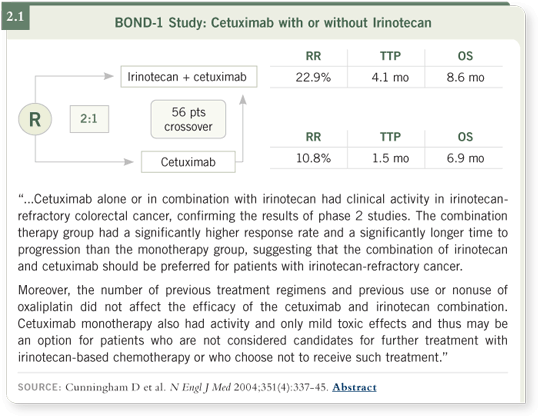
That was clever and insightful to have done at the time because there was a 23 percent response rate in the third-line setting. That kind of response rate is unheard of — second-line FOLFOX has a 10 percent response rate. They submitted the data to the FDA, and the FDA rejected the data, so they were forced to repeat that study.
The second study was a slightly larger version of the first and included about 300 refractory colon cancer patients given cetuximab. It was led by David Cunningham and is known as the BOND-1 study. It was a Phase II randomized trial in which all of the patients had experienced progression on irino-
tecan, and about 60 percent had also experienced progression on oxaliplatin
(Cunningham 2004; [2.1]). So the study included patients in the second- and
third-line settings.
This study also found a 23 percent response rate among the patients treated
with cetuximab and irinotecan and, interestingly, a 10 to 11 percent response
rate with cetuximab alone. This trial dramatically demonstrated the efficacy of
cetuximab in a refractory patient population, which led to its approval by the
FDA in the United States.
Track 5
 DR LOVE:
DR LOVE: Can you discuss what we know about panitumumab?
 DR MARSHALL: Panitumumab is another antibody that targets the epidermal
growth factor receptor (EGFR), and yet it has some important differences
from cetuximab. It is a fully humanized antibody, which one would assume
would have the advantage of reduced reactivity and therefore a reduced need
for premedication and fewer infusion reactions.
DR MARSHALL: Panitumumab is another antibody that targets the epidermal
growth factor receptor (EGFR), and yet it has some important differences
from cetuximab. It is a fully humanized antibody, which one would assume
would have the advantage of reduced reactivity and therefore a reduced need
for premedication and fewer infusion reactions.
This assumption has been borne out in the clinic. People are tolerating this
medicine from a reactivity perspective better than patients treated with cetuximab in the past.
Although we don’t have direct trials to compare panitumumab and cetuximab, panitumumab does look like an active drug in colorectal cancer. It has
produced a very impressive set of data among end-stage colon cancer patients.
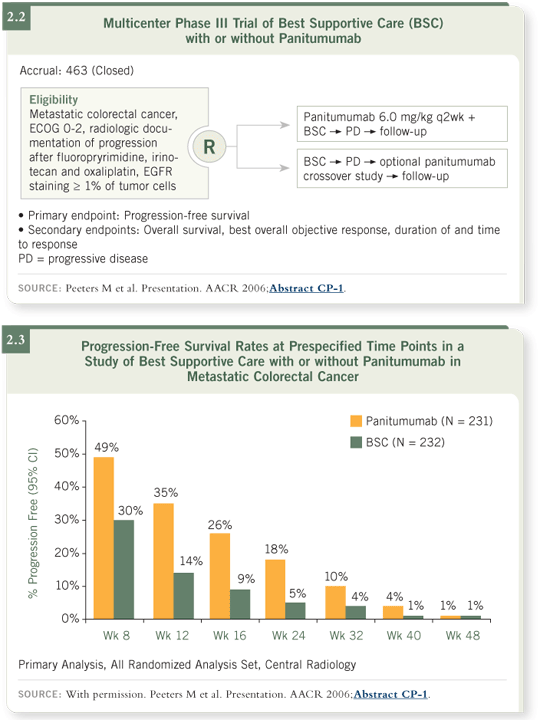
A large randomized trial comparing panitumumab to best supportive care
demonstrated approximately a 10 percent response rate as well as a significant
improvement in progression-free survival with panitumumab (Peeters 2006;
[2.2, 2.3]).
Whether this drug will play out as well in combination with chemotherapy as cetuximab has done is yet to be seen. Some small Phase I and Phase II trials of panitumumab in combination with chemotherapy look promising and consistent
(Arends 2005; Roskos 2002; Weiner 2005).
The most important study for panitumumab right now is known as the PACCE study, in which patients with colorectal cancer are treated with front-line FOLFIRI or FOLFOX with bevacizumab and then randomly assigned to panitumumab or not (Wainberg 2006; [1.3]). If that study tells us that administering
all the agents up front offers a significant advantage, then I expect we will see widespread use of that approach among patients with colon cancer.
 DR LOVE: Putting aside cost and reimbursement issues, would you use panitumumab
if it were available, and, if so, how would you integrate it into your algorithm as it relates to cetuximab?
DR LOVE: Putting aside cost and reimbursement issues, would you use panitumumab
if it were available, and, if so, how would you integrate it into your algorithm as it relates to cetuximab?
 DR MARSHALL: It’s hard to know whether we need two of these agents on the market and whether one will offer advantages over the other from a clinical perspective. Right now the two agents look very similar, but one potential difference involves the mechanism of monoclonal antibodies.
DR MARSHALL: It’s hard to know whether we need two of these agents on the market and whether one will offer advantages over the other from a clinical perspective. Right now the two agents look very similar, but one potential difference involves the mechanism of monoclonal antibodies.
Before I replace cetuximab with panitumumab, it is important to at least show equivalence in terms of efficacy between these two agents in a clinical setting.
Panitumumab offers some clear clinical advantages — it is administered every other week as opposed to weekly, it doesn’t cause infusion reactions or require premedication, and its pharmacokinetics are a little better than those of cetuximab,
but it must at least show equivalent efficacy before I would use it in place of cetuximab.
Track 6
 DR LOVE:
DR LOVE: Do you believe there is a potential clinical role for panitumumab
monotherapy?
 DR MARSHALL: Yes, I believe EGFR blockade could serve as a maintenance-type therapy. We have considered bevacizumab in that role extensively — that is, using it to prevent progression. If it weren’t for the rash associated with the EGFR agents, I believe we’d also be considering cetuximab and panitumumab in this setting.
DR MARSHALL: Yes, I believe EGFR blockade could serve as a maintenance-type therapy. We have considered bevacizumab in that role extensively — that is, using it to prevent progression. If it weren’t for the rash associated with the EGFR agents, I believe we’d also be considering cetuximab and panitumumab in this setting.
Certainly, panitumumab will likely receive its indication as monotherapy for refractory disease. Unlike cetuximab, its indication will not likely be in combination with irinotecan. Do we make the leap of faith and say it probably works just as well? I imagine we probably can, but it would be nice to have some clinical research to support that.
The question is whether panitumumab is a replacement for cetuximab and whether you’d use the panitumumab any time you would have used cetuximab.
Initially, panitumumab’s only strict indication will likely be by itself. The PACCE study will help inform us whether we can use it in other scenarios (Wainberg 2006). That raises the bigger question of when to include an EGFR blocker as a treatment option.
Right now, the indications for cetuximab and the likely indication for panitumumab
are as last therapy or, at best, second-line therapy. Agents such as bevacizumab
are leading the way as front-line treatments (Hochster 2006a, 2006b).
Should we be using these agents earlier? We still don’t have an answer for that, but the major pushback is the visible toxicity. The rash that one gets from this class of agents is a public display — and not a very attractive one — that one is on cancer therapy.
The “slippery slope” of using these agents earlier and earlier, therefore, is the rash, unless clear evidence indicates an advantage to using them early. We need efficacy data before we make that decision.
Track 7
 DR LOVE:
DR LOVE: What has your experience been with the rash and does anything help ameliorate it?
 DR MARSHALL: We are teaching patients to expect it and in some crazy way to want it, because the more rash experienced, the greater the likelihood that they are benefiting from the therapy. Some patients come back excited because they’ve developed some rash, but it is a problem. It can be very itchy, and it can be temporarily disfiguring. It would be hard to hide it in public.
DR MARSHALL: We are teaching patients to expect it and in some crazy way to want it, because the more rash experienced, the greater the likelihood that they are benefiting from the therapy. Some patients come back excited because they’ve developed some rash, but it is a problem. It can be very itchy, and it can be temporarily disfiguring. It would be hard to hide it in public.
Many people are getting dermatologists involved early in the management of the rash. I personally find that dose adjustments and modifications are the best way to manage the rash and help patients not to give up on the drug too early. The rash tends to be quite intense in the first month or two and then tends to fade and look more like a dark set of freckles.
The patients who are responding to the treatment are benefiting, and their rash tends to quiet over time and become a lot less angry looking and a lot less visible from a distance. My general recommendation is to stick it out.
 DR LOVE: How long does it take to go away, or does it go away when you stop therapy?
DR LOVE: How long does it take to go away, or does it go away when you stop therapy?
 DR MARSHALL: It goes away fairly quickly once you stop treatment — within a week or two. That is why even a week off therapy can be enough to quiet the rash.
DR MARSHALL: It goes away fairly quickly once you stop treatment — within a week or two. That is why even a week off therapy can be enough to quiet the rash.
 DR LOVE: What’s the difference between the rash with these agents compared to what you see with the tyrosine kinase inhibitors (TKIs) like erlotinib?
DR LOVE: What’s the difference between the rash with these agents compared to what you see with the tyrosine kinase inhibitors (TKIs) like erlotinib?
 DR MARSHALL: It’s a little different. These rashes are more pustular and more diffuse. I have seen cases in which the rash marches from head to foot — starting on the face and chest, moving to the body and arms, and then ultimately moving all the way down to the feet and toes. So it is an angrier rash, a little itchier and a little more persistent.
DR MARSHALL: It’s a little different. These rashes are more pustular and more diffuse. I have seen cases in which the rash marches from head to foot — starting on the face and chest, moving to the body and arms, and then ultimately moving all the way down to the feet and toes. So it is an angrier rash, a little itchier and a little more persistent.
Track 8
 DR LOVE:
DR LOVE: What do you consider to be the potential role of panitumumab in clinical practice?
 DR MARSHALL: The weekly schedule required for cetuximab is a burden, as is the need to premedicate the patient with Benadryl®. Panitumumab is administered
every two weeks, which is similar to our other regimens, and requires no loading dose and no premedication.
DR MARSHALL: The weekly schedule required for cetuximab is a burden, as is the need to premedicate the patient with Benadryl®. Panitumumab is administered
every two weeks, which is similar to our other regimens, and requires no loading dose and no premedication.
It causes no infusion reaction. If a clinician doesn’t have to worry about an allergic-type reaction or about reimbursement issues, he or she is likely to switch to panitumumab.
 DR LOVE: Are there patients for whom you might not want to combine these agents with irinotecan?
DR LOVE: Are there patients for whom you might not want to combine these agents with irinotecan?
 DR MARSHALL: Sure, some patients really do not want to try irinotecan again, but even in my practice, I’d rather administer a little irinotecan with the cetuximab or panitumumab than not use it, because the response rate is so much greater.
DR MARSHALL: Sure, some patients really do not want to try irinotecan again, but even in my practice, I’d rather administer a little irinotecan with the cetuximab or panitumumab than not use it, because the response rate is so much greater.
I can’t anticipate what the FDA will say, but I believe panitumumab has some advantages over the existing label for cetuximab because of toxicity. I believe it will have the same indication that cetuximab currently has, but perhaps with a slightly better safety profile.
Unlike cetuximab, panitumumab also has large-scale randomized data against best supportive care, so you could argue that this provides a little more proof. In addition, the results of the PACCE trial (1.3) are expected to be positive.
However, the question then arises about cost and about how you manage the disease. If the response rate is 80 to 90 percent in the front-line setting, you might be able to perform curative resections in some patients, but then what do you do with the patients?
I also believe that the combination of EGFR-VEGF blockade with no chemotherapy
might be the way to go. Patients would come in every couple of weeks and receive their antibody load.
Track 9
 DR LOVE:
DR LOVE: Can you discuss your approach to patients with Stage II disease?
 DR MARSHALL: I begin by asking why I wouldn’t administer FOLFOX chemotherapy to a patient with Stage II disease when 5-FU has shown efficacy in three percent of patients treated. I believe that adding oxaliplatin might increase efficacy by another two to three percent.
DR MARSHALL: I begin by asking why I wouldn’t administer FOLFOX chemotherapy to a patient with Stage II disease when 5-FU has shown efficacy in three percent of patients treated. I believe that adding oxaliplatin might increase efficacy by another two to three percent.
So if I believe that I can help six out of every 100 patients treated, and I present that to a patient from a risk/benefit perspective, very few patients will
turn down that chemotherapy (2.4).
This is similar to breast cancer patients, who also accept chemotherapy despite
its efficacy in only one percent of patients treated. I don’t need a 9,000-patient
clinical trial to prove that the three to six percent is real. I am in the camp of
“Why not give a patient chemotherapy?”
However, I have to recognize that I am greatly overtreating patients. I am
treating 70 to 80 out of 100 patients who can’t benefit from chemotherapy
because they don’t have cancer. Instead of an excuse to administer chemotherapy, I am looking for a good excuse to say, “You don’t need it.”
That’s why I like the current ECOG clinical trial for patients who are of
average risk — those who have had enough nodes sampled and who are not
found to have “bad things” under the microscope.
For these patients, we can then use further genetic markers to try to confirm
that. It’s a difficult trial to explain to patients and get them to enroll in, but it
is an important study.
If we can demonstrate that patients with those genetic characteristics do not
have an increased risk of recurrence, then we can save all of those people from
having to receive chemotherapy. That, to me, is the most important piece.

Select publications

