
Tracks 1-12 |
| Track 1 |
Introduction |
| Track 2 |
Identification of prognostic genes in colon cancer |
| Track 3 |
Ongoing development of a prognostic assay for colon cancer |
| Track 4 |
Significance of 18Q deletion and DCC expression |
| Track 5 |
Future directions in colon cancer based on prognostic assays |
| Track 6 |
Adjuvant chemotherapy for
Stage II colon cancer |
| Track 7 |
Rationale for incorporation of patients with Stage II disease into NSABP-C-08 |
|
| Track 8 |
Side effects associated with bevacizumab observed in NSABP-C-08 |
| Track 9 |
Efficacy and tolerability of
panitumumab |
| Track 10 |
Future directions for NSABP adjuvant colon cancer trials |
| Track 11 |
NSABP-R-04: Neoadjuvant radiation therapy and capecitabine with or without oxaliplatin versus neoadjuvant radiation therapy and infusional 5-FU in rectal cancer |
| Track 12 |
Ongoing and future trials in the NSABP |
|
|
Select Excerpts from the Interview and the CME Symposium
Track 2
 DR LOVE:
DR LOVE: Can you comment on your recent work with Genomic Health in colon cancer?
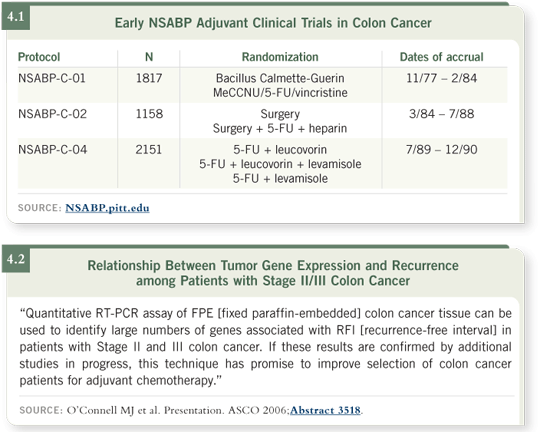
 DR O’CONNELL: We’ve gone back and looked at fixed paraffin-embedded tissue from 270 patients participating in NSABP-C-01 and NSABP-C-02, and another 300 patients participating in NSABP-C-04 (O’Connell 2006; [4.1]).
DR O’CONNELL: We’ve gone back and looked at fixed paraffin-embedded tissue from 270 patients participating in NSABP-C-01 and NSABP-C-02, and another 300 patients participating in NSABP-C-04 (O’Connell 2006; [4.1]).
The intention regarding prognostic markers in colon cancer is to provide a scientific rationale for the selection of patients with Stage II colon cancer who might benefit from adjuvant chemotherapy. We’re looking to develop a prognostic signature that would identify patients at very high risk following surgery alone, for whom you might consider adjuvant treatment, and another group who might have very low risk and would be appropriately treated with surgery alone.
 DR LOVE: Which genes were examined?
DR LOVE: Which genes were examined?
 DR O’CONNELL: Genomic Health identified 757 genes, based on review of the
medical literature, various microarray studies and consideration of molecular
pathways.
DR O’CONNELL: Genomic Health identified 757 genes, based on review of the
medical literature, various microarray studies and consideration of molecular
pathways.
It’s interesting that a number of clusters of highly interrelated genes were
identified and shown to be significantly related to outcome as measured by
recurrence-free interval in NSABP-C-01 and NSABP-C-02 (O’Connell
2006; [4.2]). These clusters of genes were quite different from the ones we
saw previously in breast cancer with the Oncotype DX assay; hence, different
molecular pathways are involved.
The second part of our project was looking for specific predictive markers that
will indicate which patients would benefit from adjuvant 5-FU/leucovorin.
We chose 300 patients from NSABP-C-04 who received 5-FU/leucovorin
and looked at the same 757 genes. The analysis is not complete, and we have
not yet identified a potential predictive signature. However, 42 or 43 genes
that showed significant correlation with outcome in NSABP-C-01 and
NSABP-C-02 also showed significant correlation with outcome in NSABP-
C-04 (O’Connell 2006).
The hazard ratios in each of these two studies were very similar, suggesting
that a cluster of genes were prognostic in both studies. A few outlying genes
showed up in NSABP-C-04 (O’Connell 2006) but didn’t show up in NSABP-
C-01/C-02, and these would be potential candidates as predictive markers,
but those analyses are ongoing.
Track 3
 DR LOVE:
DR LOVE: Were you able to categorize the patients into lower and higher risk groups?
 DR O’CONNELL: In an exploratory sense, we found, in the NSABP-C-01/C-02 data sets, that we were able to identify one third of the Stage II patients who had a recurrence rate greater than 40 percent (O’Connell 2006). If that holds up in future studies as we go through the validation process, it will be useful in clinical management.
DR O’CONNELL: In an exploratory sense, we found, in the NSABP-C-01/C-02 data sets, that we were able to identify one third of the Stage II patients who had a recurrence rate greater than 40 percent (O’Connell 2006). If that holds up in future studies as we go through the validation process, it will be useful in clinical management.
 DR LOVE: What about the other two thirds of the patients?
DR LOVE: What about the other two thirds of the patients?
 DR O’CONNELL: One subset of patients was identified as having a greater than 90 percent disease-free survival rate following surgery alone, and an intermediate
group was identified. The hazard ratios for the genes identified are as robust for colon cancer as they were for breast cancer. We’ve actually identified
more genes associated with outcome in colon cancer than we previously did in breast cancer.
DR O’CONNELL: One subset of patients was identified as having a greater than 90 percent disease-free survival rate following surgery alone, and an intermediate
group was identified. The hazard ratios for the genes identified are as robust for colon cancer as they were for breast cancer. We’ve actually identified
more genes associated with outcome in colon cancer than we previously did in breast cancer.
Track 6
 DR LOVE:
DR LOVE: Let’s discuss the clinical management of Stage II disease. What do you consider a rational approach to those patients?
 DR O’CONNELL: It’s a matter of looking at the risk-to-benefit ratio. If you have a patient with T3N0 disease who had an adequate number of lymph nodes resected — say more than 10 or 12 lymph nodes — who does not have a poorly differentiated tumor and who has no evidence of high-grade obstruction
or lymphatic or vascular invasion, the risk of relapse with surgery alone is quite low.
DR O’CONNELL: It’s a matter of looking at the risk-to-benefit ratio. If you have a patient with T3N0 disease who had an adequate number of lymph nodes resected — say more than 10 or 12 lymph nodes — who does not have a poorly differentiated tumor and who has no evidence of high-grade obstruction
or lymphatic or vascular invasion, the risk of relapse with surgery alone is quite low.
 DR LOVE: What number would you present to that type of patient?
DR LOVE: What number would you present to that type of patient?
 DR O’CONNELL: The patient I’m talking about would be at an 85 percent five-year disease-free survival rate, maybe even a little higher. I would tell the patient that we have an excellent chance — eight or nine chances out of 10 — that surgery cured the cancer and that the incremental benefit of the treatments we have available, in absolute terms, would be quite small, maybe adding one or two percent to the five-year disease-free survival.
DR O’CONNELL: The patient I’m talking about would be at an 85 percent five-year disease-free survival rate, maybe even a little higher. I would tell the patient that we have an excellent chance — eight or nine chances out of 10 — that surgery cured the cancer and that the incremental benefit of the treatments we have available, in absolute terms, would be quite small, maybe adding one or two percent to the five-year disease-free survival.
Even in this situation you need to engage the patient, because I’ve had individual patients say, “If there’s a one or a two percent potential benefit, I would like to take the treatment.” In general, however, most of the patients I talk with in this situation would choose observation.
On the other hand, if a patient has a high-grade T4 lesion with obstruction,
lymphatic or vascular invasion and only five or six lymph nodes were removed, then I would say that the risk of relapse would be closer to 30 or 35 percent. In this situation, without medical contraindication, the benefit associated with adjuvant therapy would outweigh the risk for many patients.
 DR LOVE: What type of chemotherapy would you offer to a younger patient
with Stage II disease who is in good condition and had some type of adverse
prognostic factor?
DR LOVE: What type of chemotherapy would you offer to a younger patient
with Stage II disease who is in good condition and had some type of adverse
prognostic factor?
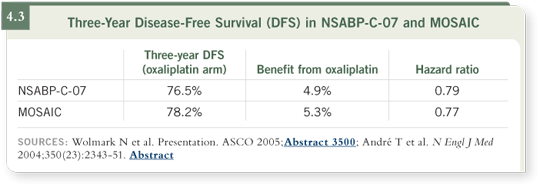
 DR O’CONNELL: For a younger patient with adverse factors, high-risk tumor
and no comorbid conditions, I would strongly advocate using the most effective regimens available, which in my view are oxaliplatin combined with
5-FU/leucovorin — either the FOLFOX regimen used in the MOSAIC study
(de Gramont 2005) or the FLOX regimen used in NSABP-C-07 (Wolmark
2005). Each of those regimens showed very similar improvements in outcome
(4.3).
DR O’CONNELL: For a younger patient with adverse factors, high-risk tumor
and no comorbid conditions, I would strongly advocate using the most effective regimens available, which in my view are oxaliplatin combined with
5-FU/leucovorin — either the FOLFOX regimen used in the MOSAIC study
(de Gramont 2005) or the FLOX regimen used in NSABP-C-07 (Wolmark
2005). Each of those regimens showed very similar improvements in outcome
(4.3).
 DR LOVE: Are you using FLOX off protocol?
DR LOVE: Are you using FLOX off protocol?
 DR O’CONNELL: I would consider it. One has to consider the overall context
of the patient. The FLOX regimen has more gastrointestinal toxicities, such
as diarrhea. The FOLFOX regimen carries a higher risk of neurotoxicity and
requires a central venous catheter and a portable ambulatory infusion pump.
DR O’CONNELL: I would consider it. One has to consider the overall context
of the patient. The FLOX regimen has more gastrointestinal toxicities, such
as diarrhea. The FOLFOX regimen carries a higher risk of neurotoxicity and
requires a central venous catheter and a portable ambulatory infusion pump.
If I had a patient with ulcerative colitis or some kind of inflammatory bowel
disease, I would not want to treat that patient with FLOX because of the
higher risk of aggravating the condition and causing severe diarrhea. If I had a
patient who was a violin player or had an underlying neuropathy, I would stay
away from the FOLFOX regimen because it includes a higher cumulative dose
of oxaliplatin.
Track 8
 DR LOVE:
DR LOVE: Would you discuss the current NSABP-C-08 adjuvant trial
for colorectal cancer, which evaluates FOLFOX with or without bevacizumab?
 DR O’CONNELL: We are pleased that our study of the use of bevacizumab in
the adjuvant setting has not shown a significant increase in toxicity. Specifically, we have not seen any increase in wound complications or in arterial
thrombotic emboli. In fact, the only toxicity we’ve seen with significantly
increased frequency is hypertension. I’m not aware of any patients who have had to stop bevacizumab because of this, and it’s been medically manageable.
DR O’CONNELL: We are pleased that our study of the use of bevacizumab in
the adjuvant setting has not shown a significant increase in toxicity. Specifically, we have not seen any increase in wound complications or in arterial
thrombotic emboli. In fact, the only toxicity we’ve seen with significantly
increased frequency is hypertension. I’m not aware of any patients who have had to stop bevacizumab because of this, and it’s been medically manageable.
One issue that was of significant potential concern was the problem of GI
perforation in patients who had recently undergone a bowel resection. At this
time, we’ve seen a total of five perforations in the trial, three in the bevacizumab arm and two in the control arm — obviously no significant difference.
 DR LOVE: How long after surgery is bevacizumab started?
DR LOVE: How long after surgery is bevacizumab started?
 DR O’CONNELL: Bevacizumab is initiated within six weeks of surgery.
DR O’CONNELL: Bevacizumab is initiated within six weeks of surgery.
 DR LOVE: In this trial design, as in the adjuvant trastuzumab studies, bevacizumab is continued for a total duration of one year.
DR LOVE: In this trial design, as in the adjuvant trastuzumab studies, bevacizumab is continued for a total duration of one year.
 DR O’CONNELL: Correct. Nobody knows the optimal duration of therapy with bevacizumab in the adjuvant setting. Our rationale in choosing one year was that we wanted to be certain bevacizumab was given the full opportunity to show a benefit, if in fact a benefit existed. If we see a significant improvement in disease-free survival, which is our primary endpoint in this trial, then it would be very appropriate to look at alternative treatment durations in the future.
DR O’CONNELL: Correct. Nobody knows the optimal duration of therapy with bevacizumab in the adjuvant setting. Our rationale in choosing one year was that we wanted to be certain bevacizumab was given the full opportunity to show a benefit, if in fact a benefit existed. If we see a significant improvement in disease-free survival, which is our primary endpoint in this trial, then it would be very appropriate to look at alternative treatment durations in the future.
Track 9
 DR LOVE:
DR LOVE: Can you talk about the potential replacement trial for NSABP-C-08?
 DR O’CONNELL: We haven’t made any decisions, but I can certainly talk about
some of the concepts we believe are interesting and quite promising. One idea
is to study FOLFOX, bevacizumab and either panitumumab or cetuximab
(4.4). In the past, NSABP has not studied anti-EGFR therapy, and we believe
that emerging data for both cetuximab and panitumumab indicate these
monoclonal antibodies may be ready for use in the adjuvant setting.
DR O’CONNELL: We haven’t made any decisions, but I can certainly talk about
some of the concepts we believe are interesting and quite promising. One idea
is to study FOLFOX, bevacizumab and either panitumumab or cetuximab
(4.4). In the past, NSABP has not studied anti-EGFR therapy, and we believe
that emerging data for both cetuximab and panitumumab indicate these
monoclonal antibodies may be ready for use in the adjuvant setting.
The Intergroup is looking at FOLFOX with or without cetuximab (N0147),
which I think is a very rational study. In fact, in the interval between our
current study and our next study, we plan to support the Intergroup trial.
Panitumumab is interesting for a number of reasons. Because it’s a fully human
monoclonal antibody and doesn’t have any murine components, no antimouse antibodies are produced by the patients. It also is associated with lower rates of
infusion reactions, and this molecule has some theoretical advantages, although
its mechanism of action is apparently very similar to cetuximab.
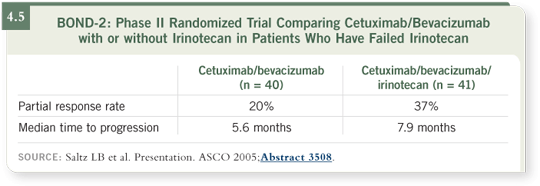
We also found the data presented by Len Saltz from the BOND-2 trial,
combining cetuximab with bevacizumab, very intriguing (Saltz 2005; [4.5]).
His preliminary data, although from relatively small numbers and a nonrandomized sequential study, were still very intriguing in that the response rates
were significantly higher when cetuximab and bevacizumab were administered together to irinotecan-refractory patients with metastatic colon cancer
— about twice as high — compared to when cetuximab was used by itself in
previous studies (Cunningham 2004; Saltz 2004).
Obviously this doesn’t establish that combined monoclonal therapy is superior,
but it hints that this may be a productive strategy to pursue. Some of the
patients also received irinotecan, and some received cetuximab and bevacizumab alone.
Of the patients who were resistant to irinotecan and then received further
irinotecan with cetuximab and bevacizumab, 37 percent responded. Of the
patients who received only the antibodies without irinotecan, the response rate
was about 20 percent (Saltz 2005; [4.5]), which is still substantial activity.
 DR LOVE: Can you also talk about the data that were recently presented
comparing panitumumab to best supportive care?
DR LOVE: Can you also talk about the data that were recently presented
comparing panitumumab to best supportive care?
 DR O’CONNELL: This was the study where more than 300 patients who
had received two or three previous chemotherapy regimens for metastatic
colorectal cancer were randomly assigned to either best supportive care or best
supportive care and panitumumab.
DR O’CONNELL: This was the study where more than 300 patients who
had received two or three previous chemotherapy regimens for metastatic
colorectal cancer were randomly assigned to either best supportive care or best
supportive care and panitumumab.
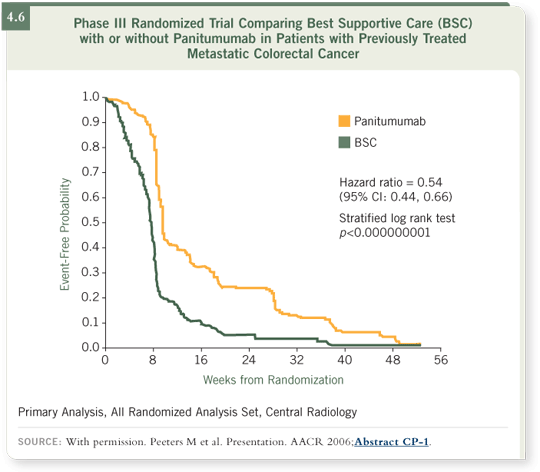
There was a marked improvement in progression-free survival, the primary
endpoint, among the patients receiving panitumumab. In fact, the progression-free survival hazard ratio was decreased by about 46 percent for patients
receiving panitumumab, and these results were highly statistically significant
(Peeters 2006; [4.6]).
We have a randomized study, looking not at tumor response rates but progression-free survival, which showed a definite biological effect. As a single agent,
panitumumab also produces objective responses in about 10 percent of patients previously treated with chemotherapy, which is very similar to what we’re
seeing with cetuximab.
 DR LOVE: What has been seen in terms of side effects and toxicity?
DR LOVE: What has been seen in terms of side effects and toxicity?
 DR O’CONNELL: The toxicity associated with panitumumab, like cetuximab,
is a cutaneous eruption — acneiform skin rash. Nearly 100 percent of the
patients receiving panitumumab are reported to have some degree of skin rash.
Infusion reactions have been very uncommon with panitumumab.
DR O’CONNELL: The toxicity associated with panitumumab, like cetuximab,
is a cutaneous eruption — acneiform skin rash. Nearly 100 percent of the
patients receiving panitumumab are reported to have some degree of skin rash.
Infusion reactions have been very uncommon with panitumumab.
A variety of other side effects are seen infrequently — diarrhea, fatigue — but
the major dose-limiting side effect has been skin rash.
 DR LOVE: To what extent, if any, has panitumumab been studied in combination with chemotherapy?
DR LOVE: To what extent, if any, has panitumumab been studied in combination with chemotherapy?
 DR O’CONNELL: A large randomized trial is currently being conducted
that’s called the PACCE trial (1.3). It’s a trial of first-line therapy for patients
with metastatic colorectal cancer. All patients receive chemotherapy, either
FOLFOX or FOLFIRI (but most of the patients receive FOLFOX in this
particular study design).
DR O’CONNELL: A large randomized trial is currently being conducted
that’s called the PACCE trial (1.3). It’s a trial of first-line therapy for patients
with metastatic colorectal cancer. All patients receive chemotherapy, either
FOLFOX or FOLFIRI (but most of the patients receive FOLFOX in this
particular study design).
All patients also receive bevacizumab. The experimental group also receives
panitumumab, so they receive dual monoclonal antibody inhibition — anti-EGFR and anti-angiogenesis therapy — with chemotherapy.
Track
11
 DR LOVE:
DR LOVE: Can you talk about where we are with the NSABP-R-04 trial?
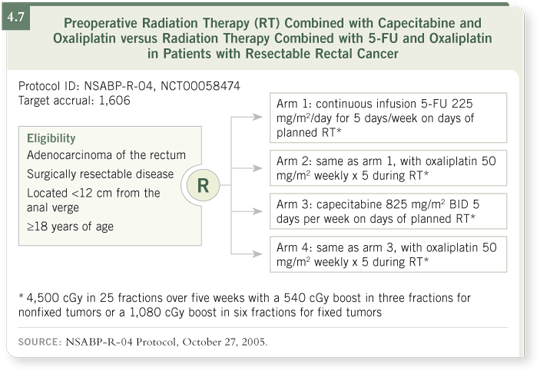
 DR O’CONNELL: NSABP-R-04 is a neoadjuvant study for patients with potentially operable carcinoma of the rectum. We offer patients neoadjuvant chemotherapy and radiation therapy, and then they go to definitive surgery. NSABP-R-04 started out comparing the standard approach of continuous infusion 5-FU during radiation therapy to a new and possibly more effective and convenient approach using capecitabine with radiation therapy.
DR O’CONNELL: NSABP-R-04 is a neoadjuvant study for patients with potentially operable carcinoma of the rectum. We offer patients neoadjuvant chemotherapy and radiation therapy, and then they go to definitive surgery. NSABP-R-04 started out comparing the standard approach of continuous infusion 5-FU during radiation therapy to a new and possibly more effective and convenient approach using capecitabine with radiation therapy.
The study has now accrued over 300 patients to examine the efficacy and toxicity profiles of capecitabine versus continuous infusion 5-FU with radiation
therapy. However, about a year ago we were also very interested in the results from a number of pilot studies that also incorporated oxaliplatin into the neoadjuvant setting.
CALGB showed a pathologic complete response rate of about 25 percent in their pilot study (Ryan 2006). Another smaller study, conducted earlier by ECOG, also showed preliminary but interesting results (Rosenthal 2003).
Therefore, we’ve modified NSABP-R-04 to make it a factorial design. In addition to receiving either capecitabine or continuous infusion 5-FU, half of the patients also receive oxaliplatin. The study has four individual treatments in a two-by-two factorial design (4.7). That addendum has been sent out to our membership recently; it has been activated and should be coming on line very soon.
 DR LOVE: What do we know about the side effects and tolerability of the fluoropyrimidines with oxaliplatin and radiation therapy for rectal cancer?
DR LOVE: What do we know about the side effects and tolerability of the fluoropyrimidines with oxaliplatin and radiation therapy for rectal cancer?
 DR O’CONNELL: What we know comes from these pilot studies (Ryan 2006; Rosenthal 2003). Severe diarrhea is a potential side effect when we administer
high-dose pelvic irradiation combined with a fluorinated pyrimidine and oxaliplatin. In fact, problems with severe diarrhea in the pilot studies required some modification in our Phase III trial.
DR O’CONNELL: What we know comes from these pilot studies (Ryan 2006; Rosenthal 2003). Severe diarrhea is a potential side effect when we administer
high-dose pelvic irradiation combined with a fluorinated pyrimidine and oxaliplatin. In fact, problems with severe diarrhea in the pilot studies required some modification in our Phase III trial.
We decreased the number of doses of oxaliplatin in the Phase III trial by one dose because of this increase in toxicity. Also, we advocate careful monitoring and, if the patient develops diarrhea during treatment, interrupting the weekly doses of oxaliplatin and the fluorinated pyrimidine. So the major side effect we’re concerned about is diarrhea with dehydration, which will require very careful monitoring.
 DR LOVE: Do you think oxaliplatin has a role in the neoadjuvant setting off protocol right now?
DR LOVE: Do you think oxaliplatin has a role in the neoadjuvant setting off protocol right now?
 DR O’CONNELL: One could make that argument. It’s an active drug in this disease. We haven’t proven that it will add to the treatment benefit, but we know oxaliplatin-containing regimens for patients with metastatic disease have
definitely increased response rates and improved survival. So I believe one
could make a rationale for doing that. If the clinician decides to do that, great
caution should be exercised because of the potential for greater toxicities.
DR O’CONNELL: One could make that argument. It’s an active drug in this disease. We haven’t proven that it will add to the treatment benefit, but we know oxaliplatin-containing regimens for patients with metastatic disease have
definitely increased response rates and improved survival. So I believe one
could make a rationale for doing that. If the clinician decides to do that, great
caution should be exercised because of the potential for greater toxicities.
Select publications

