
Tracks 1-13 |
| Track 1 |
Introduction |
| Track 2 |
Side-effect profile of bevacizumab |
| Track 3 |
Bowel perforation associated
with bevacizumab |
| Track 4 |
Bevacizumab-related hypertension |
| Track 5 |
Predictive risk factors for arterial thromboembolic events with bevacizumab |
| Track 6 |
Potential rationale for lack of benefit with adjuvant irinotecan |
| Track 7 |
Impact of exercise and diet
on cancer relapse |
|
| Track 8 |
Selection of adjuvant chemotherapy |
| Track 9 |
Role of capecitabine in the adjuvant setting |
| Track 10 |
Substitution of capecitabine for 5-FU as neoadjuvant therapy
for rectal cancer |
| Track 11 |
First-line therapy for metastatic disease |
| Track 12 |
Sequencing panitumumab in the clinical algorithm |
| Track 13 |
Potential benefits and challenges of combined biologic therapy |
|
|
Select Excerpts from the Interview and the CME Symposium
Track 2
 DR LOVE:
DR LOVE: Would you comment on the side effects associated with bevacizumab and their implications for the adjuvant setting?
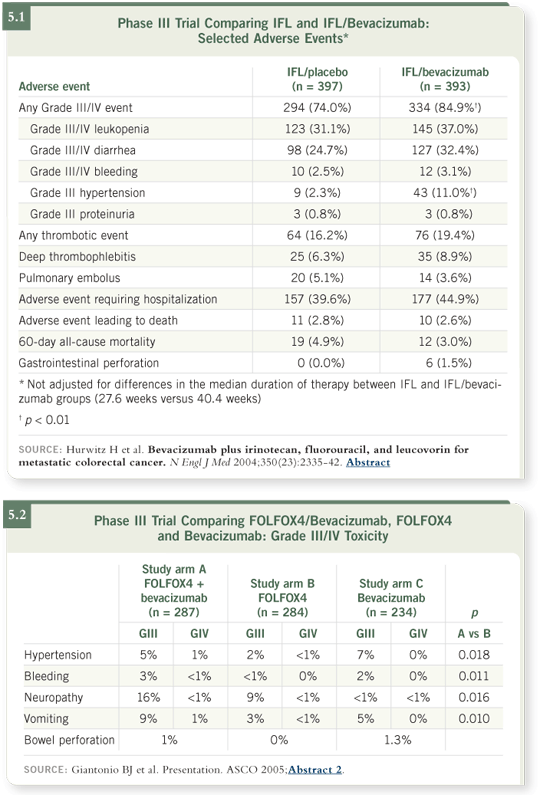
 DR HURWITZ: The side-effect profile for bevacizumab is best established from the large Phase III studies in advanced disease with the IFL regimen (Hurwitz 2004; [5.1]) and also the FOLFOX4 regimen (Giantonio 2005; [5.2]). In general, several themes to the toxicity profile in those settings may be informative
about what to expect in the adjuvant setting.
DR HURWITZ: The side-effect profile for bevacizumab is best established from the large Phase III studies in advanced disease with the IFL regimen (Hurwitz 2004; [5.1]) and also the FOLFOX4 regimen (Giantonio 2005; [5.2]). In general, several themes to the toxicity profile in those settings may be informative
about what to expect in the adjuvant setting.
The first is that no increase occurred in the chemotherapy-related side effects. The only exception was an increase in neuropathy, which was related to a greater time on oxaliplatin because patients were deriving more benefit and staying on treatment longer (Giantonio 2005; [5.2]). This should not be an increased risk in the adjuvant setting, because the duration of treatment is mandated by the standards of adjuvant therapy, not by continued benefit as in the advanced disease setting.
Aside from the chemotherapy-related side effects, which are not increased, a few bevacizumab-specific side effects may be relevant in the adjuvant setting. Most notably, these are going to be issues like hypertension. In general, however, this is probably reversible over time for most patients.
A small increase appears in the risk of an arterial thromboembolic event, such as a heart attack, stroke, transient ischemic attack or unstable angina. While there are many ways to look at this in aggregate, the best way is probably to assume that the risk is increased about twofold (Skillings 2005). The vast majority of such events are not fatal, although even nonfatal events can carry significant morbidity.
When the background rate is one percent, which is the risk for most patients with advanced colorectal cancer, doubling that risk goes to about two percent or so. If the background risk is one to two percent, the risk with bevacizumab is around three to four percent.
In the adjuvant setting, this may be an issue for patients who would potentially
be cured, if a small increased risk of morbidity were related to these arterial thromboembolic events. In general for advanced disease, the greatest risk to the patient in terms of both mortality and morbidity is the cancer. In the adjuvant setting, the risk associated with arterial thromboembolic events is likely to be low.
However, we don’t know the true risk-to-benefit ratio until we know the impact of adjuvant bevacizumab in terms of improvement in disease-free and overall survival, the incidence of specific side effects — such as arterial thromboembolic
events — and the implications of those side effects on mortality or morbidity.
Track 3
 DR LOVE:
DR LOVE: What’s the incidence of bowel perforation associated with bevacizumab?
 DR HURWITZ: Bowel perforation occurs in about one to two percent of patients treated with bevacizumab, particularly in settings that include a risk of some inflammatory condition or injury to the bowel. For example, patients with colorectal cancer have a risk of perforation around one to 1.5 percent (Hurwitz 2004; [5.1]; Giantonio 2005; [5.2]).
DR HURWITZ: Bowel perforation occurs in about one to two percent of patients treated with bevacizumab, particularly in settings that include a risk of some inflammatory condition or injury to the bowel. For example, patients with colorectal cancer have a risk of perforation around one to 1.5 percent (Hurwitz 2004; [5.1]; Giantonio 2005; [5.2]).
It’s not clear if these events are tied to any one special phenomenon. For example, they do not always occur in the setting of chemotherapy enteritis. Sometimes they’re associated with a procedure; sometimes the inciting event is not clear. This risk also does not appear to be related to having cancer in situ. The bigger risk, in terms of perforation, is wound healing in general. It’s hard to predict exactly what the event rate will be in the adjuvant setting, although we do have some guidance from patients with more advanced disease.
If one looks at patients with advanced or metastatic disease who were treated
after they healed from surgery and waited at least four weeks, one sees no
increased risk in wound healing complications if they were on bevacizumab as compared to chemotherapy alone. By extrapolation, in the adjuvant setting, most patients will probably not see any increased risk of wound healing.
In addition, because patients are, in general, a little healthier in the adjuvant than in the metastatic setting, it is plausible that the overall risk profile may even be better in this group of patients. What we don’t know is whether some long-term complications may be seen only after long surveillance in patients who are cured of their cancer. In all likelihood, such risks, if any, are likely to be small, but we can’t speculate at this point what they may actually be.
Track 4
 DR LOVE:
DR LOVE: Does the hypertension usually reverse upon discontinuation of bevacizumab?
 DR HURWITZ: Hypertension seems to be reversible in most patients. The reversal of hypertension after the discontinuation of bevacizumab, as one would expect, has not been well studied. This is in part because patients who have discontinued bevacizumab come off protocol, and their follow-up is obviously limited.
DR HURWITZ: Hypertension seems to be reversible in most patients. The reversal of hypertension after the discontinuation of bevacizumab, as one would expect, has not been well studied. This is in part because patients who have discontinued bevacizumab come off protocol, and their follow-up is obviously limited.
Anecdotally, it appears that most patients will have their hypertension reduced, if not completely normalized, over a period of several weeks to months — probably consistent with the half-life of the drug.
Track 5
 DR LOVE:
DR LOVE: Do models exist to predict the baseline risk of arterial
thromboembolic events?
 DR HURWITZ: The considerations related to the risk of arterial thromboembolic
events, in one part, should be seen as those that are standard risks for cardiovascular disease. Most cardiovascular studies, including intervention
studies, have excluded patients with cancer, so we don’t know whether typical risks in noncancer populations extrapolate to cancer populations. This becomes even more complicated because of all the other issues going on in cancer patients.
DR HURWITZ: The considerations related to the risk of arterial thromboembolic
events, in one part, should be seen as those that are standard risks for cardiovascular disease. Most cardiovascular studies, including intervention
studies, have excluded patients with cancer, so we don’t know whether typical risks in noncancer populations extrapolate to cancer populations. This becomes even more complicated because of all the other issues going on in cancer patients.
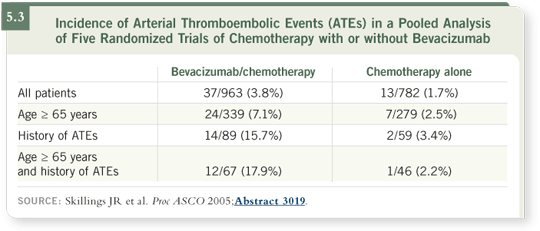
Within the study of irinotecan/5-FU/leucovorin with or without bevacizumab
and several other studies, a model has been created to try to identify which risk factors may predict who is at higher risk of an arterial thromboembolic
event (Skillings 2005; [5.3]). At this point, that appears to be patients who are older and those who have had a prior event.
Patients with an arterial thromboembolic event more than a year prior to enrollment were allowed to participate in the clinical studies. However, a prior event more than a year before and an age of 65 years or more do appear to increase the risk for an arterial thromboembolic event in general and in particular with bevacizumab (Skillings 2005).
Track 8
 DR LOVE:
DR LOVE: Can you review your clinical algorithm for adjuvant therapy in
patients with Stage II or Stage III disease?
 DR HURWITZ: For Stage III disease, the two approaches that I believe are
currently well supported are the adjuvant use of FOLFOX or, for patients who
are not candidates for oxaliplatin, capecitabine alone. It is a logical interpolation to use capecitabine and oxaliplatin instead of FOLFOX, given the activity
of capecitabine alone. However, that is an extrapolation that has not yet been
validated.
DR HURWITZ: For Stage III disease, the two approaches that I believe are
currently well supported are the adjuvant use of FOLFOX or, for patients who
are not candidates for oxaliplatin, capecitabine alone. It is a logical interpolation to use capecitabine and oxaliplatin instead of FOLFOX, given the activity
of capecitabine alone. However, that is an extrapolation that has not yet been
validated.
In the adjuvant setting, with potential curative intent, I’m a bit more reluctant
to treat patients outside a standard, well-proven algorithm. Therefore, I usually
use FOLFOX for most robust patients. Since FOLFOX is tolerated fairly well
by most patients, we usually start with this even for those who are older,
unless they have a clear contraindication to oxaliplatin, such as a significant
preexisting neuropathy.
For patients with Stage II disease, the discussion is much more complicated
because it’s not clear that any standard therapy exists. A benefit has been
suggested but has not yet been proven. For those patients, the risk-to-benefit
discussion can often take a considerable amount of time to put it in a frame of
reference that’s meaningful.
For example, the risk reduction for a patient with Stage II disease may be an
absolute difference of one or two percent. Also, we’re not sure whether that
one or two percent benefit exists. It could be three to four percent, but it’s not
likely to be more than a five percent absolute difference.
I believe there can be room for discussion with patients about whether a few
percent difference in overall survival is worth six months of therapy. I usually
have that discussion with them, and then I take their lead on how they prioritize the risks and benefits in their individual setting.
We need to remember the adequacy of lymph node sampling in the discussion about Stage II versus Stage III disease. We know the data fairly well for those with properly staged Stage II or Stage III disease. Those who are “stage ambiguous,” with less than 11 lymph nodes, have a prognosis that sits between Stage II and Stage III disease.
While we don’t know for sure that treatment will have a similar benefit in that population, it’s a reasonable inference. Therefore, for patients who have inadequate lymph-node staging, the discussion about the benefits of adjuvant therapy is probably a little bit more favorable than for those who have true Stage II disease.
Track 9
 DR LOVE:
DR LOVE: In breast cancer, a concept has evolved of using less toxic and probably slightly less effective chemotherapy for patients with node-negative tumors. Typically, those patients might receive an anthracycline without a taxane. Do you think the same approach would make sense in colon cancer, for example, using capecitabine for patients with Stage II disease?
 DR HURWITZ: The approach makes sense in general but carries a few caveats. The first is that we need data to drive the discussion. One of the problems is that when we believe we should be able to extrapolate a result to a different setting, it doesn’t always work.
DR HURWITZ: The approach makes sense in general but carries a few caveats. The first is that we need data to drive the discussion. One of the problems is that when we believe we should be able to extrapolate a result to a different setting, it doesn’t always work.
Another issue is whether the substitution of capecitabine for FOLFOX is being made in a hybrid or a rational way. That is, if one believes that a benefit truly exists in the adjuvant setting with FOLFOX for patients with Stage II disease — and as we discussed, a small benefit might exist — it’s not clear that a benefit exists with capecitabine.
It clearly will be a little better tolerated; however, if it’s not active, the risk-to-benefit ratio may not be as favorable. Therefore, I tend not to do that.
Track 12
 DR LOVE:
DR LOVE: Where do you believe panitumumab will fit into the treatment of colorectal cancer, and what is your opinion about the data that have been reported so far?
 DR HURWITZ: Panitumumab showed benefit in the third-line setting compared to best supportive care (Peeters 2006; [2.3]). It causes tumor shrinkage that as monotherapy is in the same ballpark as cetuximab. The tumor control rate showed improvement. Evaluating the curves as a whole, the hazard ratio is favorable. No survival benefit emerged in that study (Peeters 2006).
DR HURWITZ: Panitumumab showed benefit in the third-line setting compared to best supportive care (Peeters 2006; [2.3]). It causes tumor shrinkage that as monotherapy is in the same ballpark as cetuximab. The tumor control rate showed improvement. Evaluating the curves as a whole, the hazard ratio is favorable. No survival benefit emerged in that study (Peeters 2006).
The studies of panitumumab with chemotherapy are not as mature as those with cetuximab. While a strong likelihood exists that the two antibodies will perform similarly, we need the data to validate that. The monotherapy third-line setting with panitumumab will be highly appropriate. Its use with chemotherapy earlier on must be driven by the data as they mature.
Track 13
 DR LOVE:
DR LOVE: What are your thoughts about combining biologic therapies,
specifically bevacizumab and an EGFR inhibitor?
 DR HURWITZ: The combination of VEGF and EGF inhibition makes sense,
particularly if you are a mouse. For patients, the data are unfortunately
limited. In colorectal cancer, we do have the promising pilot study known as
BOND-2, which is cetuximab/irinotecan/bevacizumab versus cetuximab/bevacizumab (Saltz 2005; [4.5]). The original BOND-1 study compared
cetuximab alone or with irinotecan (Cunningham 2004).
DR HURWITZ: The combination of VEGF and EGF inhibition makes sense,
particularly if you are a mouse. For patients, the data are unfortunately
limited. In colorectal cancer, we do have the promising pilot study known as
BOND-2, which is cetuximab/irinotecan/bevacizumab versus cetuximab/bevacizumab (Saltz 2005; [4.5]). The original BOND-1 study compared
cetuximab alone or with irinotecan (Cunningham 2004).
There are many problems with cross-study comparisons. Also, disclaimers
have been made for data from relatively small studies in which the therapeutic
regimen was attractive, meaning that the potential exists for bias in the types
of patients who were accrued.
Saltz reported the study combining cetuximab and bevacizumab, and in
general, those patients seem to be similar to the types of patients accrued to
previous studies, although other imbalances may not have been detected. The
response rates and times to progression with the double biologics were impressive (Saltz 2005; [4.5]).
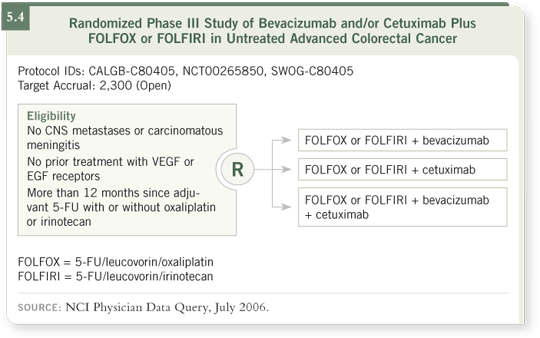
One pilot study and the wonderful mouse data we mentioned justify
combining these drugs in clinical trials. That is why the GI Intergroup’s first-line study (C80405) is chemotherapy (FOLFOX or FOLFIRI) with cetuximab
or bevacizumab or both (5.4). It is also the reason for the first-line PACCE study, which compares FOLFOX/bevacizumab with or without panitumumab (1.3). A number of studies will be needed to address this question.
Select publications

