
Tracks 1-9 |
| Track 1 |
Introduction |
| Track 2 |
Background, design and results of TREE-1 and TREE-2 trials |
| Track 3 |
Efficacy of bFOL regimen in TREE-1 and TREE-2 |
| Track 4 |
Selection of first-line therapy for metastatic disease |
| Track 5 |
Management of patients with neurotoxicity who progress on FOLFOX with bevacizumab |
|
| Track 6 |
Management of oxaliplatin-associated neurotoxicity |
| Track 7 |
Selection of first-line therapy for patients who have received adjuvant FOLFOX |
| Track 8 |
Potential benefits of panitumumab
versus cetuximab |
| Track 9 |
Future directions in the management of colon cancer |
|
|
Select Excerpts from the Interview and the CME Symposium
Tracks 2-4
 DR LOVE:
DR LOVE: What was the rationale for the TREE trials?
 DR HOCHSTER: I proposed the TREE study around the time that oxaliplatin was approved. The real issue at that time was whether it was necessary to administer 5-fluorouracil (5-FU) by infusion or if it could be administered on a bolus schedule, which we piloted at NYU, or the schedule used with capecitabine reported in the European literature.
DR HOCHSTER: I proposed the TREE study around the time that oxaliplatin was approved. The real issue at that time was whether it was necessary to administer 5-fluorouracil (5-FU) by infusion or if it could be administered on a bolus schedule, which we piloted at NYU, or the schedule used with capecitabine reported in the European literature.
We didn’t want to mount a huge study that would look for outcome differences,
because frankly, we didn’t expect a large difference in terms of response rate or survival between the three arms: infusional 5-FU (modified FOLFOX6), bolus 5-FU (bFOL) and capecitabine (CAPOX). We did predict, however, that a significant difference in toxicity might appear among the arms, and we definitely anticipated differences in convenience.
So we designed a randomized trial to compare toxicity among the three arms over the first 12 weeks. Although this trial is referred to as a Phase II trial, it really is a Phase III trial in how it is designed and the endpoint it evaluates (Hochster 2005; [3.1]).
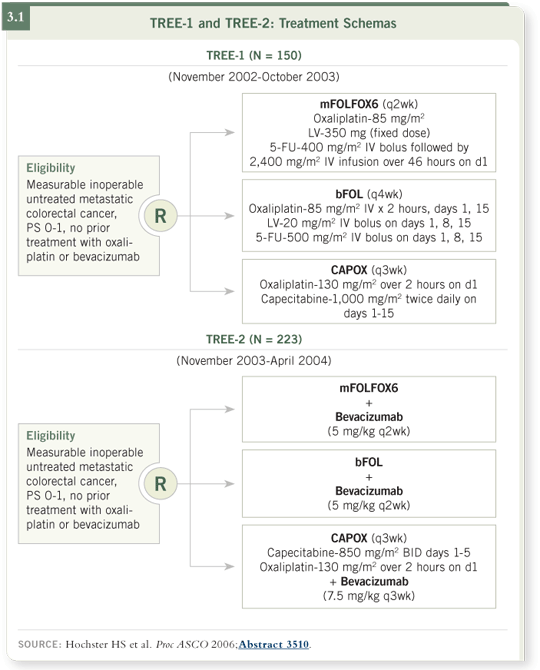
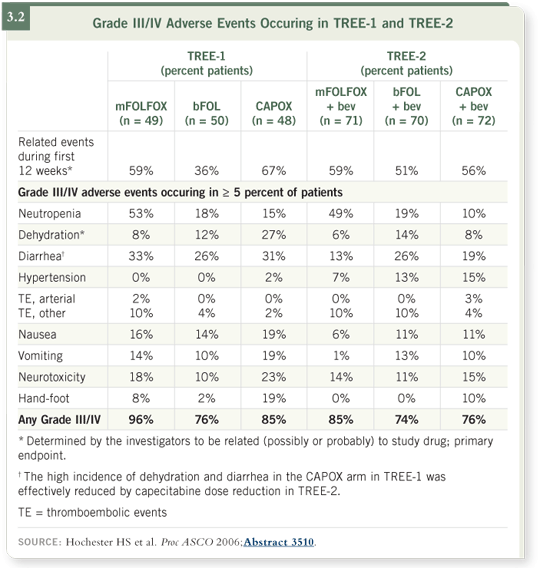
With 50 patients per arm, the study found about a 25 percent difference in the
overall incidence of Grade III-IV toxicity between the 5-FU infusion arm and
the 5-FU bolus arm (3.2).
The study was interesting because it showed that initially the worst-tolerated
regimen was CAPOX when administered according to the European schedule
at 1,000 mg/m2 twice a day. This group experienced a lot more diarrhea,
leukopenia and hospitalizations, and 50 percent of the patients required dose
reduction within the first 12 weeks. Based on that finding, the Data Safety
Monitoring Board (DSMB), which independently reviewed the toxicity data,
suggested that further trials should use the reduced dose of capecitabine of
850 mg/m2 twice per day.
During this time in 2004, the data were just being reported on the efficacy of
bevacizumab with bolus IFL (Hurwitz 2004), and FOLFOX was beginning to
be used as the first-line regimen of choice based on the Intergroup NCCTG-N9741 study (Grothey 2004).
Based on this, we decided to amend the TREE protocol by adding bevacizumab to each arm so that we could quickly obtain toxicity data on each of
these oxaliplatin-fluoropyrimidine combinations together with the anti-VEGF
antibody.
The study was amended and opened in essentially the same institutions, with
the addition of a couple more, and we then treated 70 patients per arm, so
we had about 220 patients in the TREE-2 cohort (Hochster 2006a; [3.3]).
So we had two cohorts within the TREE protocol that were sequential in
time, which is a historical comparison but a very good historical comparison
given that the studies included virtually the same investigators and the same
protocol.
When assessing overall toxicity among the regimens, we found that CAPOX
with bevacizumab was now tolerated well with the reduced capecitabine dose
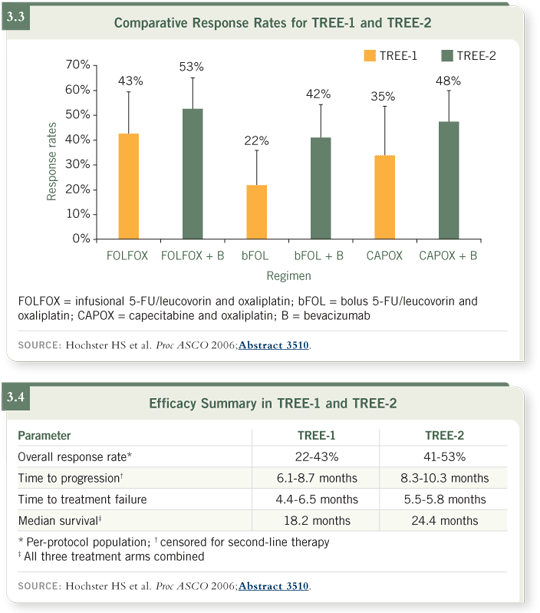
as well as the FOLFOX with bevacizumab regimen (3.2). The response rates
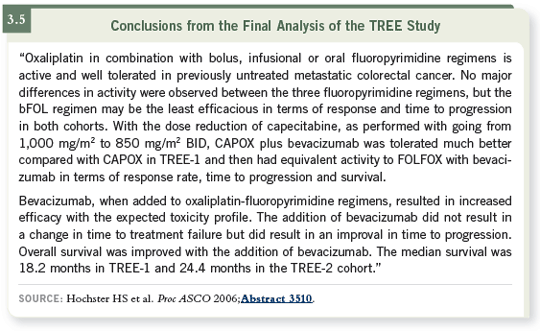
were higher with the addition of bevacizumab (3.3, 3.4, 3.5).
 DR LOVE: Can you discuss the update of the data reported at ASCO?
DR LOVE: Can you discuss the update of the data reported at ASCO?
 DR HOCHSTER: With bevacizumab, the time to progression was approximately two to three months longer for each of the arms, especially for the
CAPOX arm (Hochster 2006).
DR HOCHSTER: With bevacizumab, the time to progression was approximately two to three months longer for each of the arms, especially for the
CAPOX arm (Hochster 2006).
Survival is more difficult to comment on because we still haven’t had 50 percent deaths in each of the arms in the TREE-2 cohort after well over two years. It’s fair to say that the median overall survival will be greater than two years in the TREE-2 trial, with the addition of bevacizumab to the treatment regimens.
Comparison of these regimens shows that the bevacizumab-specific toxicities
were no different — that is, the incidences of hemorrhagic and thrombotic
complications were similar to those reported previously. By and large, the data
are comparable to what would have been expected based on first- and second-
line data from ECOG-E3200 (Giantonio 2005).
I don’t see that any surprises have come out of the TREE-2 study. It simply
shows that all of these regimens are well tolerated in the first-line setting and
that you can add bevacizumab without significantly changing the toxicity of
the chemotherapy. The anti-angiogenesis type toxicity is pretty much the same
across regimens.
 DR LOVE: What about the combination of CAPOX with bevacizumab?
DR LOVE: What about the combination of CAPOX with bevacizumab?
 DR HOCHSTER: The CAPOX regimen is based on these data using the lowest
doses of capecitabine, so CAPOX combined with bevacizumab is an equally
good regimen as far as we can see in terms of toxicity and activity. I believe
this regimen is a reasonable choice.
DR HOCHSTER: The CAPOX regimen is based on these data using the lowest
doses of capecitabine, so CAPOX combined with bevacizumab is an equally
good regimen as far as we can see in terms of toxicity and activity. I believe
this regimen is a reasonable choice.
Approval for the use of bevacizumab with 5-FU-based therapy that doesn’t
incorporate capecitabine may be the one issue with using this regimen, but
if it’s not a reimbursement issue, then I believe that’s a reasonable option as
well. I’m not sure that this regimen is a big favor for a lot of patients because
it requires them to take the pills twice a day for 14 days compared to simply
being on a pump for 48 hours.
 DR LOVE: Why do you use FOLFOX with bevacizumab rather than irinotecan or FOLFIRI with bevacizumab?
DR LOVE: Why do you use FOLFOX with bevacizumab rather than irinotecan or FOLFIRI with bevacizumab?
 DR HOCHSTER: The oxaliplatin-based chemotherapy is tolerated pretty well
by most patients. Patients experience some fatigue, but we can easily control the nausea. Hair loss is minimal, and we see much less diarrhea than with irinotecan.
DR HOCHSTER: The oxaliplatin-based chemotherapy is tolerated pretty well
by most patients. Patients experience some fatigue, but we can easily control the nausea. Hair loss is minimal, and we see much less diarrhea than with irinotecan.
I’ve personally been impressed with the responses I’ve seen with oxaliplatin compared to irinotecan, so I tend to use oxaliplatin-based therapy, but it’s hard to be dogmatic about that. If a patient has issues with neuropathy or some other difficulty that makes them not want oxaliplatin, then irinotecan-based therapy is a perfectly reasonable option.
Track 5
 DR LOVE:
DR LOVE: How do you treat a patient who has a good response to FOLFOX with bevacizumab but then develops neuropathy and has to discontinue the oxaliplatin?
 DR HOCHSTER: At the time that the patient develops the neurotoxicity, I stop the oxaliplatin and continue the 5-FU and leucovorin regimen with bevacizumab.
On average, these patients will go another four to six months before they eventually have disease progression. At that point, I hope a study will be open that I can recommend to them.
DR HOCHSTER: At the time that the patient develops the neurotoxicity, I stop the oxaliplatin and continue the 5-FU and leucovorin regimen with bevacizumab.
On average, these patients will go another four to six months before they eventually have disease progression. At that point, I hope a study will be open that I can recommend to them.
Outside of a study, however, the number of choices one has is pretty large. You can give irinotecan alone, administer FOLFIRI or continue bevacizumab at that point. You can give cetuximab, which would be outside the labeled indication for this drug, or you can administer the chemotherapy first followed by irinotecan and cetuximab.
A lot of options are available. In general, it depends on the patient. For a very robust, younger patient whom I’m trying to treat with everything possible, I’d probably continue with FOLFIRI and bevacizumab and then go to cetuximab as third-line treatment.
I believe this treatment approach provides patients an extra opportunity for response. If their disease progresses, then I’ll give bevacizumab and cetuximab together, based on the data from the BOND study (Cunningham 2004; Saltz 2005).
 DR LOVE: If the neuropathy is starting to resolve, will you reintroduce oxaliplatin?
DR LOVE: If the neuropathy is starting to resolve, will you reintroduce oxaliplatin?
 DR HOCHSTER: I do try to go back to oxaliplatin down the line. Many times we can get people out to maybe a two-year interval by using irinotecan followed by irinotecan and cetuximab, and patients can go on for a couple of years. At that point, I normally go back to oxaliplatin.
DR HOCHSTER: I do try to go back to oxaliplatin down the line. Many times we can get people out to maybe a two-year interval by using irinotecan followed by irinotecan and cetuximab, and patients can go on for a couple of years. At that point, I normally go back to oxaliplatin.
Sometimes we have to deal with hypersensitivity reactions to oxaliplatin, especially on the second dose, but by and large patients seem to benefit again from going back on oxaliplatin. Their disease is at least stabilized, and some patients have nice responses the second time on oxaliplatin.
Select publications

