| Section D: Clinical trial participation |
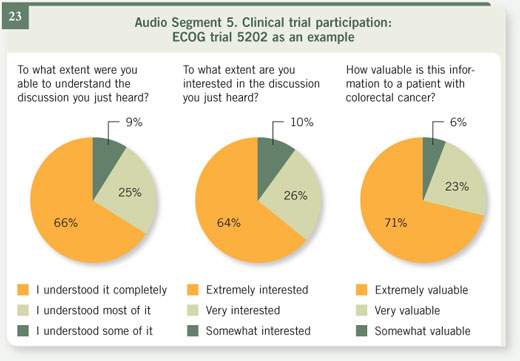
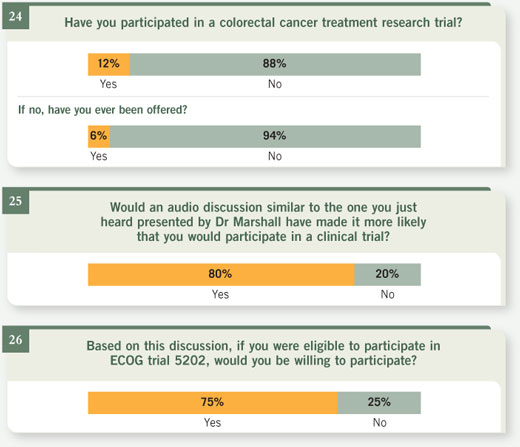
  Select excerpts from audio segment 5 Select excerpts from audio segment 5
 DR LOVE: After listening to you discuss some of the statistics for adjuvant
chemotherapy, it seems that decisions for patients with Stage II disease are
particularly challenging. Can you talk about some of the research that’s
going on to try to improve therapy for these patients? DR LOVE: After listening to you discuss some of the statistics for adjuvant
chemotherapy, it seems that decisions for patients with Stage II disease are
particularly challenging. Can you talk about some of the research that’s
going on to try to improve therapy for these patients? |
 DR MARSHALL: If you’re faced with Stage II disease, you recognize that
maybe 70 to 80 percent of the time, you’re getting chemotherapy and going
through all the hassle and the risk, and you don’t need it. What we need is to
be smarter about who should get the chemotherapy and who shouldn’t. If we could figure out which group of patients is at the higher risk, the bad quarter
of the patients, if you will, and give only that group of patients chemotherapy,
that would be a major advance. DR MARSHALL: If you’re faced with Stage II disease, you recognize that
maybe 70 to 80 percent of the time, you’re getting chemotherapy and going
through all the hassle and the risk, and you don’t need it. What we need is to
be smarter about who should get the chemotherapy and who shouldn’t. If we could figure out which group of patients is at the higher risk, the bad quarter
of the patients, if you will, and give only that group of patients chemotherapy,
that would be a major advance.
This is done with some diseases. For example, with breast cancer, it’s fairly
routine to obtain a molecular profile of the tumor to understand more about
an individual patient’s cancer and make decisions based on that profile. We
have now finally moved toward such a world in colon cancer in the form of a
major new and very large study for patients with Stage II colon cancer.
The first step in this study, which is being run through the ECOG, or
Eastern Cooperative Oncology Group, is that the individual patient’s tumor is
analyzed for genetic characteristics. If the results suggest a favorable prognosis,
we believe we should leave that patient alone and not administer the chemotherapy,
because our best guess is that the patient is in the good three quarters
of the patients. And therefore, only those patients with the bad cancer genetics
will receive chemotherapy.
 DR LOVE: Can any oncologist enter a patient on this study? DR LOVE: Can any oncologist enter a patient on this study?
 DR MARSHALL: It’s an Intergroup study, so it’s being done all across the
country at most major cancer centers and should be accessible through a
variety of clinicians. So most patients should be able to get ahold of this study. DR MARSHALL: It’s an Intergroup study, so it’s being done all across the
country at most major cancer centers and should be accessible through a
variety of clinicians. So most patients should be able to get ahold of this study.
 DR LOVE: So part of entering this study is that the patient’s tumor is studied
with new specialized tests? DR LOVE: So part of entering this study is that the patient’s tumor is studied
with new specialized tests?
 DR MARSHALL: That’s right. Before a decision is made about whether to treat
an individual patient, the genetics of the patient’s tumor are studied. DR MARSHALL: That’s right. Before a decision is made about whether to treat
an individual patient, the genetics of the patient’s tumor are studied.
 DR LOVE: Is that an advantage of being in the study, that these tests are done
on a patient’s tumor? DR LOVE: Is that an advantage of being in the study, that these tests are done
on a patient’s tumor?
 DR MARSHALL: It is one of the only ways you can get the test done. So it
absolutely is an advantage, because if someone gives me a hint that my tumor
is more favorable and I don’t need chemotherapy, that’s a nice thing to hear. DR MARSHALL: It is one of the only ways you can get the test done. So it
absolutely is an advantage, because if someone gives me a hint that my tumor
is more favorable and I don’t need chemotherapy, that’s a nice thing to hear.
 DR LOVE: What happens if the test shows a less favorable prognosis? DR LOVE: What happens if the test shows a less favorable prognosis?
 DR MARSHALL: That part of the study involves a randomization — and
I’ll tell you what that means — between the standard FOLFOX that we’ve
discussed and FOLFOX with a new medicine, bevacizumab, or Avastin, added
to it. In other words, this study is looking not only at finding the right patient
to receive chemotherapy but also at whether adding bevacizumab to the
FOLFOX will further improve a patient’s outcome. DR MARSHALL: That part of the study involves a randomization — and
I’ll tell you what that means — between the standard FOLFOX that we’ve
discussed and FOLFOX with a new medicine, bevacizumab, or Avastin, added
to it. In other words, this study is looking not only at finding the right patient
to receive chemotherapy but also at whether adding bevacizumab to the
FOLFOX will further improve a patient’s outcome.
Now, randomization often spooks patients. But in this setting, for example,
there is no placebo. Everyone’s receiving the standard of care, FOLFOX. But
half of the patients also receive the bevacizumab. Randomization is done by a
computer, which assigns each patient to one of the two treatments.
 DR LOVE: And, in fact, isn’t it true that all of the treatments that we’ve been
talking about today have been studied in these large randomized trials? DR LOVE: And, in fact, isn’t it true that all of the treatments that we’ve been
talking about today have been studied in these large randomized trials?
 DR MARSHALL: That’s right. The only reason we have the new medicines
that our patients today enjoy is because the generation or so of colon cancer
patients who came before them put themselves into clinical trials to move the
bar, to improve the outcome for everyone. DR MARSHALL: That’s right. The only reason we have the new medicines
that our patients today enjoy is because the generation or so of colon cancer
patients who came before them put themselves into clinical trials to move the
bar, to improve the outcome for everyone.
I really feel it’s an obligation not only for us, as doctors, but for our patients
as well to carry that torch of clinical research and get questions answered as
quickly as we can so that our children can enjoy the benefits of the research
that we do today.
|


