
Tracks 1-18 |
| Track 1 |
Introduction |
| Track 2 |
Developing long-term strategies
for the surgical and systemic
treatment of metastatic disease |
| Track 3 |
Management of patients with
synchronous primary and
metastatic disease |
| Track 4 |
Impact of age on management
of synchronous primary and
metastatic disease |
| Track 5 |
Time course for surgery after
preoperative bevacizumab |
| Track 6 |
Hepatic resection, ablation or
the combination for hepatic
metastases |
| Track 7 |
Applying oncologic “judgment”
to management of hepatic
metastases |
| Track 8 |
Clinical use of hepatic arterial
infusion |
| Track 9 |
Necessity of developing criteria
and standards for hepatic
resection |
| Track 10 |
CAPOX versus FOLFOX in the
adjuvant and metastatic settings |
|
| Track 11 |
Comparability of capecitabine
and continuous infusion 5-FU
in chemoradiation regimens for
rectal cancer |
| Track 12 |
Convenience of neoadjuvant
capecitabine versus infusional
5-FU for rectal cancer |
| Track 13 |
Role of downstaging clinical Stage
III disease in selection of adjuvant
chemotherapy |
| Track 14 |
Clinical trial of preoperative
capecitabine/bevacizumab with
radiation therapy in rectal cancer |
| Track 15 |
Potential mechanisms of action
of bevacizumab |
| Track 16 |
Incorporation of oxaliplatin into
neoadjuvant chemoradiation
therapy for rectal cancer |
| Track 17 |
CONFIRM-1: FOLFOX with or
without vatalanib as first-line
therapy |
| Track 18 |
Effects of bevacizumab and
chemotherapeutic agents
on the liver |
|
|
Select Excerpts from the Interview
 Track 6
Track 6
 DR LOVE:
DR LOVE: Will you discuss the surgical treatment of colorectal metastases
to the liver?
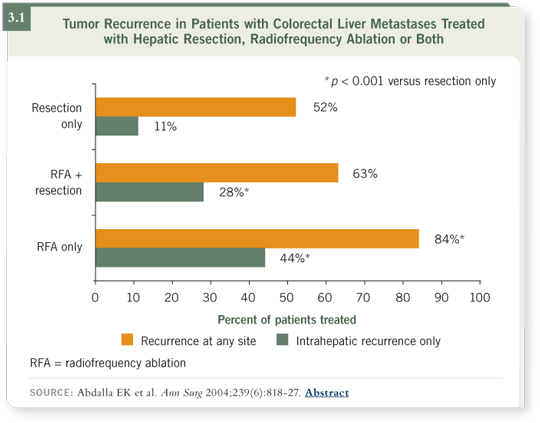
 DR WOLFF: Steve Curley has published data for patients who have undergone hepatic resection, hepatic resection combined with radiofrequency ablation or
ablation only as the means to approach the metastatic component in the liver
(Abdalla 2004). The patients who did best had a resection, and the patients
who did worse had ablation only. Patients who had a combination of resection
and ablation have intermediate results (3.1). So we’re fans of resection
whenever possible, if that’s technically doable and we have enough hepatic
remnant.
DR WOLFF: Steve Curley has published data for patients who have undergone hepatic resection, hepatic resection combined with radiofrequency ablation or
ablation only as the means to approach the metastatic component in the liver
(Abdalla 2004). The patients who did best had a resection, and the patients
who did worse had ablation only. Patients who had a combination of resection
and ablation have intermediate results (3.1). So we’re fans of resection
whenever possible, if that’s technically doable and we have enough hepatic
remnant.
 Track 10
Track 10
 DR LOVE:
DR LOVE: What are your thoughts about using CAPOX as adjuvant treatment
for colon cancer?
 DR WOLFF: Enough data are available now that most authorities would say
CAPOX and FOLFOX are almost equivalent. If they are not exactly the same
in terms of efficacy, they are pretty close.
DR WOLFF: Enough data are available now that most authorities would say
CAPOX and FOLFOX are almost equivalent. If they are not exactly the same
in terms of efficacy, they are pretty close.
If we were talking about 5-FU versus capecitabine, a lot of physicians would
favor capecitabine over a 5-FU program. This is an individual physician and
patient choice, but I personally don’t have any problems using capecitabine
with oxaliplatin as part of the standard adjuvant treatment.
The logic would be that capecitabine is equivalent to 5-FU and leucovorin
in the adjuvant setting, possibly a little bit better, and certainly less toxic.
FOLFOX is better than 5-FU and leucovorin in the adjuvant setting and, therefore, I believe CAPOX is a reasonable adjuvant substitute.
 DR LOVE: What about using CAPOX with bevacizumab?
DR LOVE: What about using CAPOX with bevacizumab?
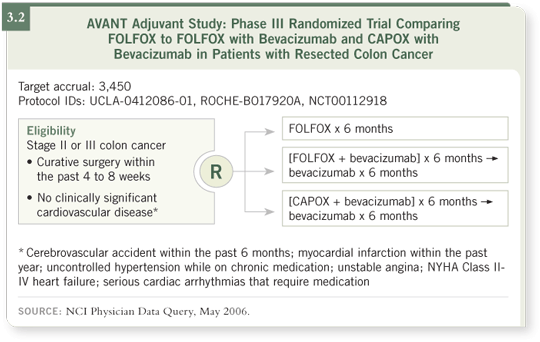
 DR WOLFF: That’s a very attractive program, and this is a question the
AVANT trial (3.2) will evaluate in the adjuvant setting: Would CAPOX
with bevacizumab be equivalent to FOLFOX with bevacizumab? The most
interesting question is whether bevacizumab adds anything in the adjuvant
setting. Theoretically, if bevacizumab is an anti-angiogenic therapy, you might
question how much benefit that drug could give you in the setting of microscopic
metastatic disease.
DR WOLFF: That’s a very attractive program, and this is a question the
AVANT trial (3.2) will evaluate in the adjuvant setting: Would CAPOX
with bevacizumab be equivalent to FOLFOX with bevacizumab? The most
interesting question is whether bevacizumab adds anything in the adjuvant
setting. Theoretically, if bevacizumab is an anti-angiogenic therapy, you might
question how much benefit that drug could give you in the setting of microscopic
metastatic disease.
Lee Ellis has been a strong proponent that bevacizumab is not simply anti-angiogenic
therapy; it’s anti-VEGF therapy (Hicklin 2005; Ellis 2005). Data
indicate that VEGF receptors are present on the tumor cells (Zhang 2002) and
that VEGF may act as an autocrine growth factor for the tumor itself (Masood
2001).
So if you inhibit that pathway, you may obtain a greater benefit in the
adjuvant setting. It will be interesting to see the data. Anybody who attempts
to predict the result of that trial may be surprised. A difference may exist
between bevacizumab and no bevacizumab, but I’ll be surprised if a difference
appears between FOLFOX/bevacizumab and CAPOX/bevacizumab.
 Track 11
Track 11
 DR LOVE:
DR LOVE: Another situation in which capecitabine may be used in place
of continuous-infusion 5-FU is the neoadjuvant treatment of rectal cancer
with radiation therapy. What are your thoughts about that?
 DR WOLFF: That’s a very good question. One of the issues we need to recognize
is that we can’t study everything. We have to identify which research
questions are the most important to answer.
DR WOLFF: That’s a very good question. One of the issues we need to recognize
is that we can’t study everything. We have to identify which research
questions are the most important to answer.
I personally don’t believe the question of whether capecitabine is superior,
inferior or equivalent to infusional 5-FU or bolus 5-FU is so important to
answer.
The Memorial group has data from preoperative rectal cancer demonstrating
that whether you give patients bolus 5-FU with radiation therapy or infusional
5-FU with radiation therapy, the outcomes are equivalent.
The surprise came with the Intergroup trial, which showed infusional 5-FU
as part of adjuvant therapy for rectal cancer was superior to bolus 5-FU
(O’Connell 1994). Part of that may have been related to how much of the
agent the patients in the bolus 5-FU arm received.
But if we accept the Memorial data — indicating no difference between bolus
5-FU and radiation therapy and infusional 5-FU with radiation therapy as
part of a preoperative strategy — it’s very likely that capecitabine will not be
inferior to infusional or bolus 5-FU.
 Track 14
Track 14
 DR LOVE:
DR LOVE: Would you discuss the neoadjuvant trial for patients with rectal
cancer that you are currently conducting?
 DR WOLFF: We are studying a combination of capecitabine, administered
daily with bevacizumab, which will be administered every two weeks during
the course of radiation therapy. Patients receive bevacizumab and capecitabine
starting on the first day of the radiation and then receive bevacizumab every
two weeks through radiation. After six weeks of rest, they are referred back to
the surgeons, at which point the tumor is reevaluated.
DR WOLFF: We are studying a combination of capecitabine, administered
daily with bevacizumab, which will be administered every two weeks during
the course of radiation therapy. Patients receive bevacizumab and capecitabine
starting on the first day of the radiation and then receive bevacizumab every
two weeks through radiation. After six weeks of rest, they are referred back to
the surgeons, at which point the tumor is reevaluated.
This trial opened fairly recently. I’ve just had a patient complete it, and her
disease has been downstaged from T3/N1 to T2/N0.
Select publications

