
The highly informative and frequently entertaining National Surgical
Adjuvant Breast and Bowel Project (NSABP) group meetings have always been
among my favorite oncologic events. Over the years, I have spent many hours
in the audience at these conferences listening intently to the discussion of ideas
and concepts that would ultimately change the face of cancer treatment.
In the 80s and 90s Dr Bernard Fisher, who in the minds of many is the father
of randomized clinical trials in cancer treatment, chaired these meetings and
led the NSABP in a number of bold new directions. Today, Dr Norman
Wolmark nobly carries forth this tradition of innovation, and the group’s latest
concept for their next adjuvant colon cancer trial (C-11), discussed at their
most recent meeting in Denver the last weekend in April, exemplifies this
tradition (1.1).
One of the most impressive aspects of the NSABP is its unique ability to get
things done and done well. The group’s trials ask simple yet critical questions
and obtain answers expeditiously. The aforementioned new adjuvant trial
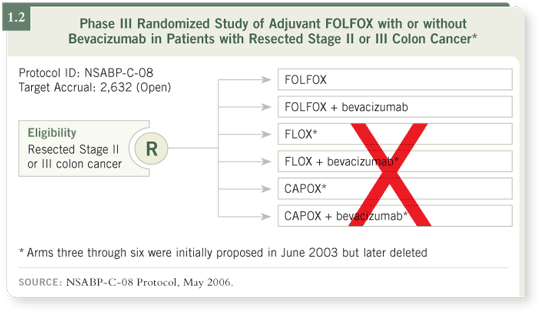
proposed in Denver follows NSABP-C-08, which started out as a glimmer
in Dr W’s eye in June 2003 at the group’s meeting in Orlando (1.2).
At that time, what was so impressive about the C-08 concept was that
Dr Aimery de Gramont had presented the adjuvant FOLFOX data just a
few weeks earlier at ASCO. Yet there was the NSABP — whose leadership
anticipated the positive MOSAIC trials results — ready to take action.
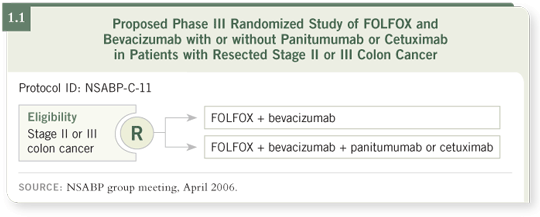
The proposed C-11 design is similarly forward thinking. Consider for
a moment that the groundbreaking data in advanced colorectal cancer
comparing panitumumab — a highly interesting humanized anti-EGFR
monoclonal antibody — to best supportive care followed by panitumumab
on progression had just been presented at the AACR meeting in Washington,
DC a few weeks previously. Nonetheless, the NSABP’s Dr Michael O’Connell
was up at the podium considering adding this exciting agent to the presumed
superior regimen in C-08.
Of even greater and certainly more immediate interest, at the meeting in
Denver, Dr Wolmark updated the group on the status of C-08 and estimated
that the trial will complete accrual in September! Having efficiently entered
more than 2,000 people in about two years, the son of FOLFOX is ready for a
new sibling.
The spectacular results of the adjuvant trastuzumab breast cancer trials
— including NSABP-B-31 — have suddenly raised our hopes that the future
of oncology lies in a new generation of targeted treatment options that will
provide major steps forward.
One particularly interesting aspect of trials like C-08 and C-11 is that patients
have the opportunity to receive promising therapies — like bevacizumab,
cetuximab and panitumumab — that would otherwise not be available to
them in the adjuvant setting.
Although there can never be a guarantee of either safety or benefit, patients
facing a significant risk of relapse despite our best interventions will eagerly
embrace this new generation of trials. In fact, our CME group’s recent survey
of 150 colon cancer survivors demonstrated that 75 percent would
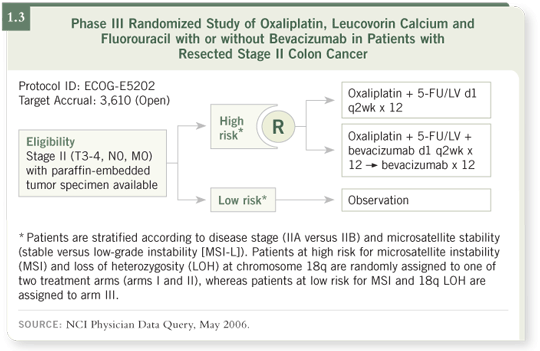
have been willing (if eligible) to enter ECOG trial 5202 for patients with
Stage II tumors (1.3), which evaluates the prognostic value of microsatellite
instability, 18q deletions and FOLFOX with or without bevacizumab. (Now
there’s a familiar concept!)
Who could have imagined that in the span of 36 months, adjuvant therapy for
colon cancer would have evolved from the old warhorse, 5-FU/leucovorin,
to testing regimens that include a platinum compound, two biologic agents
and an oral fluoropyrimidine prodrug?…The NSABP, that’s who. Their next
meeting is in Baltimore in October. More to come.
— Neil Love, MD
NLove@ResearchToPractice.net
Select publications

