
Tracks 1-19 |
| Track 1 |
Introduction |
| Track 2 |
X-ACT adjuvant trial evaluating
capecitabine versus the Mayo
Clinic regimen |
| Track 3 |
European and United States
dosing of capecitabine |
| Track 4 |
X-ACT trial: Efficacy and side-effect
data |
| Track 5 |
Management of capecitabine- associated
side effects |
| Track 6 |
Clinical use of adjuvant
capecitabine monotherapy for
colon cancer |
| Track 7 |
Patient perspectives on the value
of benefits from adjuvant therapy |
| Track 8 |
Substitution of capecitabine for
infusional 5-FU |
| Track 9 |
Impact of alternate schedules of
capecitabine on tolerability |
| Track 10 |
Incorporating biologic agents into
adjuvant clinical trials |
|
| Track 11 |
Clinical benefit of adding bevacizumab
to chemotherapy for
metastatic disease |
| Track 12 |
Continuation of bevacizumab
after disease progression |
| Track 13 |
Use of aggressive surveillance
for earlier detection of potentially
curable disease |
| Track 14 |
Role of monoclonal antibodies
targeting the EGFR in colorectal
cancer |
| Track 15 |
Efficacy and tolerability of panitumumab
monotherapy |
| Track 16 |
Difficulties in evaluating newly
emerging agents in colorectal
cancer |
| Track 17 |
Identification of predictors of
response in colorectal cancer |
| Track 18 |
Need for predictive assays in
colorectal cancer |
| Track 19 |
Development of oral tyrosine
kinase inhibitors in breast and
colon cancer |
|
|
Select Excerpts from the Interview
 Track 3
Track 3
 DR LOVE:
DR LOVE: The difference in dosing of capecitabine in European versus
North American studies has provoked much discussion. What did you
observe in the X-ACT trial with the full dose of 2,500 mg/m
2 per day (in
two doses, 14 days on, seven days off )?
 DR BURRIS: A noticeable difference in dose reductions appeared between
Europe and the United States, with far fewer dose reductions in Europe. If you look at the trial as a whole, about 40 percent of the patients received a dose
reduction. For American patients, the percentage was much higher.
DR BURRIS: A noticeable difference in dose reductions appeared between
Europe and the United States, with far fewer dose reductions in Europe. If you look at the trial as a whole, about 40 percent of the patients received a dose
reduction. For American patients, the percentage was much higher.
Of the patients enrolled from our institution, almost 80 percent had their
dose reduced. The fact that a higher percentage of Americans received a dose
reduction may reflect that Americans have more leucovorin in their bodies
because they are so well fed and well folated.
No variable other than location — including age, sex or size of the patients
— was related to differences in dose reduction. American doctors were very
quick to reduce dose at any sign of toxicity, and I believe they are certainly
comfortable with lower doses of 2,000 mg/m2. In the curves for the trial, the
patients whose doses were reduced did just as well as those who remained at
the full dose.
 Track 4
Track 4
 DR LOVE:
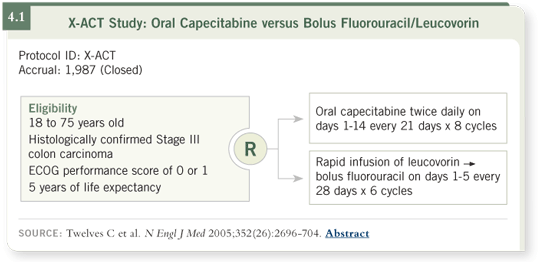
DR LOVE: Can you summarize the efficacy findings in the X-ACT
study (4.1)?
 DR BURRIS: Although it was designed to show noninferiority, the trial nearly
showed superiority of capecitabine in the various endpoints studied. Time to
relapse was significantly superior for capecitabine at the standard p-value of
0.05. A nonsignificant trend toward improved survival was also seen, with a p-value of 0.07.
DR BURRIS: Although it was designed to show noninferiority, the trial nearly
showed superiority of capecitabine in the various endpoints studied. Time to
relapse was significantly superior for capecitabine at the standard p-value of
0.05. A nonsignificant trend toward improved survival was also seen, with a p-value of 0.07.
For all three endpoints studied — disease-free, relapse-free and overall survival
— capecitabine was about 15 percent better than the 5-FU arm (4.2). This
translates into a small, incremental, four to five percent absolute benefit in
each of those endpoints. So it was a “win” for capecitabine in that regard. In
addition, because the p-values were powered for superiority and the trial was
designed only to show noninferiority, this led to the FDA label expansion and
the approval for capecitabine as adjuvant treatment for colon cancer.
Side effects and toxicity in the capecitabine arm were primarily diarrhea and
hand-foot syndrome. Approximately 10 to 12 percent of patients experienced
Grade III toxicities, but the side effects were quickly ameliorated by changes
in dose and some dose delays.
 Track 11
Track 11
 DR LOVE:
DR LOVE: Clinically, can you detect a difference in the quality of
responses you have observed with bevacizumab/chemotherapy compared
to chemotherapy alone?
 DR BURRIS: I’ve seen tremendous responses with the addition of bevacizumab,
with near normalization or normalization of the CEAs, indicating the
contribution of VEGF inhibition and possibly improved chemotherapy permeability
to the tumors. Even more impressive is the degree of response. I’ve
noticed in looking at CAT scans that 80 to 90 percent of the tumor is gone in
those patients treated with the addition of bevacizumab to other agents, such
as capecitabine, or regimens, such as FOLFOX.
DR BURRIS: I’ve seen tremendous responses with the addition of bevacizumab,
with near normalization or normalization of the CEAs, indicating the
contribution of VEGF inhibition and possibly improved chemotherapy permeability
to the tumors. Even more impressive is the degree of response. I’ve
noticed in looking at CAT scans that 80 to 90 percent of the tumor is gone in
those patients treated with the addition of bevacizumab to other agents, such
as capecitabine, or regimens, such as FOLFOX.
 DR LOVE: What’s your typical first-line chemotherapy in a patient who’s
never had chemotherapy?
DR LOVE: What’s your typical first-line chemotherapy in a patient who’s
never had chemotherapy?
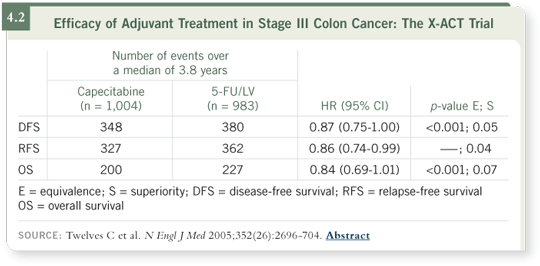
 DR BURRIS: My typical front-line regimen is CAPOX with bevacizumab. If
a patient walked into my clinic today, he or she would receive both oxaliplatin
and bevacizumab every other week and capecitabine one week on, one week
off (4.3). That regimen has gone well, with patients generally telling me that
they feel good in the second week of therapy.
DR BURRIS: My typical front-line regimen is CAPOX with bevacizumab. If
a patient walked into my clinic today, he or she would receive both oxaliplatin
and bevacizumab every other week and capecitabine one week on, one week
off (4.3). That regimen has gone well, with patients generally telling me that
they feel good in the second week of therapy.
 Track 12
Track 12
 DR LOVE:
DR LOVE: What is your approach to a patient who has an initial response
with that regimen but has stopped oxaliplatin as a result of neuropathy and
then has disease progression?
 DR BURRIS: For the most part, those patients receive irinotecan-based therapy
with bevacizumab if they are strongly EGFR-positive. I routinely test for this
in patients with colon cancer, much like testing for estrogen receptor/progesterone
receptor status in patients with breast cancer, so we have the EGFR
data on our colon cancer patients from the beginning. If a patient is strongly
EGFR-positive, I administer cetuximab with irinotecan.
DR BURRIS: For the most part, those patients receive irinotecan-based therapy
with bevacizumab if they are strongly EGFR-positive. I routinely test for this
in patients with colon cancer, much like testing for estrogen receptor/progesterone
receptor status in patients with breast cancer, so we have the EGFR
data on our colon cancer patients from the beginning. If a patient is strongly
EGFR-positive, I administer cetuximab with irinotecan.
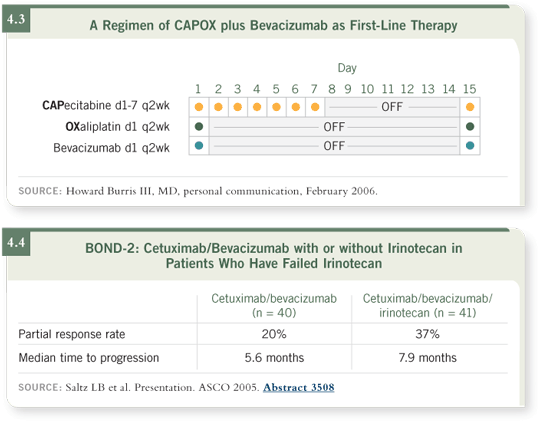
 DR LOVE: What about the combination of irinotecan, cetuximab and bevacizumab
in that situation?
DR LOVE: What about the combination of irinotecan, cetuximab and bevacizumab
in that situation?
 DR BURRIS: The data for that triplet are very encouraging (Saltz 2005;
[4.4]).
DR BURRIS: The data for that triplet are very encouraging (Saltz 2005;
[4.4]).
Select publications

