
Tracks 1-15 |
| Track 1 |
Introduction |
| Track 2 |
Selection of adjuvant
chemotherapy for colorectal
cancer |
| Track 3 |
Use of capecitabine in the
adjuvant and metastatic settings |
| Track 4 |
Dose and schedule of
capecitabine |
| Track 5 |
Clinical use of capecitabine in the
adjuvant setting |
| Track 6 |
Management of oxaliplatin-associated
neurotoxicity |
| Track 7 |
Similarities and differences
between panitumumab and
cetuximab |
| Track 8 |
Potential advantages of adjuvant
CAPOX compared to FOLFOX |
|
| Track 9 |
Staging and treatment of patients
with rectal cancer |
| Track 10 |
Selection of chemotherapy to
combine with radiation therapy in
the treatment of rectal cancer |
| Track 11 |
Clinical algorithm for first-line
therapy in patients without prior
systemic therapy |
| Track 12 |
Clinical implications of TREE-1
and TREE-2 trial results |
| Track 13 |
Continuation of bevacizumab
after disease progression |
| Track 14 |
Therapeutic approach to patients
with isolated hepatic metastasis |
| Track 15 |
Future directions in the
development of biologic agents in
colorectal cancer |
|
|
Select Excerpts from the Interview
 Track 2
Track 2
 DR LOVE:
DR LOVE: What are the clinical implications of the MOSAIC adjuvant
trial data?
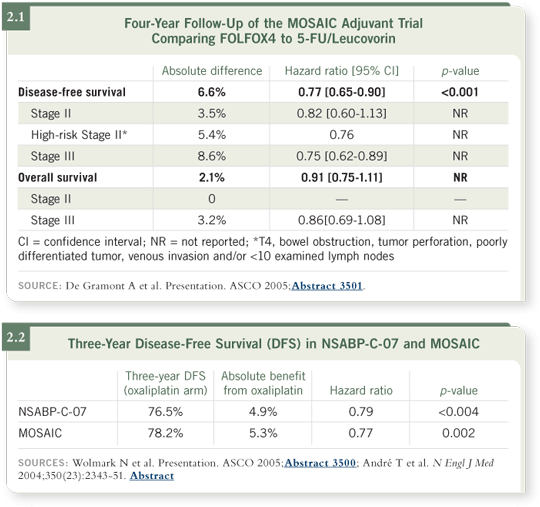
 DR CHU: It’s clear that FOLFOX certainly provides significant clinical benefit
to patients with Stage III disease, for whom oxaliplatin-based chemotherapy
is FDA approved, but I also believe, based on the MOSAIC trial data, that
patients with Stage II disease benefit significantly from FOLFOX (André
2004; de Gramont 2005; [2.1]).
DR CHU: It’s clear that FOLFOX certainly provides significant clinical benefit
to patients with Stage III disease, for whom oxaliplatin-based chemotherapy
is FDA approved, but I also believe, based on the MOSAIC trial data, that
patients with Stage II disease benefit significantly from FOLFOX (André
2004; de Gramont 2005; [2.1]).
At last year’s ASCO meeting, Norm Wolmark presented the results from
the NSABP adjuvant C-07 study, which demonstrated that a bolus 5-FU/
leucovorin/oxaliplatin regimen — FLOX — also seemed to confer significant clinical benefit (Wolmark 2005). We only have three-year disease-free
survival data, but if one looks at the improvement with the bolus regimen
of FLOX versus the infusional regimen of FOLFOX, they are virtually
identical (2.2).
So for patients with a good performance status and very few comorbid
illnesses, an oxaliplatin-based regimen is my first choice for adjuvant therapy.
For patients who are older and may have comorbid illnesses, the feeling is they
might experience increased toxicity from oxaliplatin-based chemotherapy. In
that setting fluoropyrimidine monotherapy is reasonable. Based on the results
from the X-ACT trial (Twelves 2005), an oral fluoropyrimidine in the form of
capecitabine is effective. If anything, based on the X-ACT trial, it appears to
be more active and provide more clinical benefit than 5-FU/leucovorin, with
a significantly improved safety profile.
 Tracks 3-4
Tracks 3-4
 DR LOVE:
DR LOVE: How do you approach the dosing of capecitabine in the
adjuvant and metastatic settings?
 DR CHU: In the adjuvant setting, as per the X-ACT trial (Twelves 2005),
the dose of capecitabine initially was 1,250 mg/m2 twice a day on days one
through 14 every 21 days. As it turned out, up to 42 percent of the patients
required a dose reduction during the course of the trial. It’s important to
emphasize that approximately the same number of patients who were on
5-FU/leucovorin also required a dose reduction.
DR CHU: In the adjuvant setting, as per the X-ACT trial (Twelves 2005),
the dose of capecitabine initially was 1,250 mg/m2 twice a day on days one
through 14 every 21 days. As it turned out, up to 42 percent of the patients
required a dose reduction during the course of the trial. It’s important to
emphasize that approximately the same number of patients who were on
5-FU/leucovorin also required a dose reduction.
In the metastatic setting, where we’re not attempting cure but rather palliation,
the general experience has been to start patients at a lower dose — 900
to 1,000 mg/m2 twice a day (for 14 of 21 days). In combination with either
irinotecan or oxaliplatin, at least in the United States, the standard dose we’re
now thinking about is 800 to 850 mg/m2 twice a day on days one through 14
every 21 days.
 Track 5
Track 5
 DR LOVE:
DR LOVE: What is your approach to patients with Stage II disease?
 DR CHU: It is not too different from my approach to patients with Stage III
disease. If you look at the clinical studies and the analyses conducted thus
far, there is growing evidence that adjuvant therapy for patients with Stage
II colon cancer confers benefit, although the benefit is less than that seen in
Stage III disease.
DR CHU: It is not too different from my approach to patients with Stage III
disease. If you look at the clinical studies and the analyses conducted thus
far, there is growing evidence that adjuvant therapy for patients with Stage
II colon cancer confers benefit, although the benefit is less than that seen in
Stage III disease.
Out of every 100 patients with Stage II disease whom we treat, probably at
most two to four patients may benefit, and among patients with Stage III
disease, probably six to eight would benefit. In my practice, I tend to be fairly
aggressive and have, in fact, offered FOLFOX to patients with Stage II disease.
Recently, I was referred a patient who was a music professor at our university.
They were concerned about the possibility of oxaliplatin-associated neuropathy
as this individual was a pianist, so we used capecitabine.
 Track 12
Track 12
 DR LOVE:
DR LOVE: Would you discuss the TREE-1 and TREE-2 studies and their
implications for clinical practice?
 DR CHU: The TREE-1 study was initially developed by Howard Hochster
at NYU to evaluate the toxicity, safety profile and clinical activity of three
different oxaliplatin-based regimens (Hochster 2005). One regimen was a
modified FOLFOX-6. Another regimen was bolus 5-FU/leucovorin in
combination with oxaliplatin that Howard developed, which had shown very
promising results in the Phase II setting (Hochster 2003). The third regimen
in TREE-1 was capecitabine with oxaliplatin.
DR CHU: The TREE-1 study was initially developed by Howard Hochster
at NYU to evaluate the toxicity, safety profile and clinical activity of three
different oxaliplatin-based regimens (Hochster 2005). One regimen was a
modified FOLFOX-6. Another regimen was bolus 5-FU/leucovorin in
combination with oxaliplatin that Howard developed, which had shown very
promising results in the Phase II setting (Hochster 2003). The third regimen
in TREE-1 was capecitabine with oxaliplatin.
When it became evident that bevacizumab was going to be approved by
the FDA, the trial was then modified to the TREE-2 study. It involved the
same three arms of oxaliplatin-based chemotherapy with the addition of
bevacizumab.
At the 2005 ASCO meeting, Howard reported that the bolus schedule was
inferior in clinical activity and was associated with increased toxicity. The
modified FOLFOX-6 and the CAPOX regimens were nearly identical, at least
in terms of response rate, although it did seem that the modified FOLFOX-6
was slightly better (Hochster 2005; [2.3]).
 DR LOVE: How are you approaching the selection of capecitabine versus 5-FU
in combination with bevacizumab and oxaliplatin?
DR LOVE: How are you approaching the selection of capecitabine versus 5-FU
in combination with bevacizumab and oxaliplatin?
 DR CHU: The TREE-2 study provides the rationale for substituting
capecitabine for infusional 5-FU, and we’re generally using CAPOX and
bevacizumab.
DR CHU: The TREE-2 study provides the rationale for substituting
capecitabine for infusional 5-FU, and we’re generally using CAPOX and
bevacizumab.

Select publications

