You are here: Home: CCU 4 | 2005: Philip
A Philip, MD, PhD
| Philip A Philip,
MD, PhD |
EDITED
COMMENTS |
 Role of adjuvant capecitabine: Implications
of the X-ACT trial Role of adjuvant capecitabine: Implications
of the X-ACT trial
The X-ACT adjuvant trial confirmed what was already known
about capecitabine — it is as good as or even better
than 5-FU/leucovorin (Cassidy 2004, 2005; Twelves 2005).
The X-ACT adjuvant trial also informed us that we should
be using capecitabine as an active chemotherapy agent. However,
it’s not an oral agent that we can administer to a
patient and then forget about. The adjuvant dose we start
is 2.5 g/m2 per day (days one to 14 of a 21-day cycle), not
2 g/m2 per day as we use for metastatic disease.
In situations in which a single-agent fluoropyrimidine
is being used or contemplated, capecitabine should be used.
I don’t believe at this time that, if given the option,
a patient will opt for intravenous treatment unless an issue
arises regarding who will pay for the capecitabine. Capecitabine
should be the drug of choice for patients who will receive
a single-agent fluoropyrimidine, because it’s easier
to administer and doesn’t interfere much with the patient’s
daily routine. It has side effects, and we have to pay attention
to them. But overall, it’s a treatment that patients
will prefer.
In which patients should we use single-agent therapy? In
patients with Stage III disease, the data on adjuvant FOLFOX
have completely transformed my practice (Andre 2004). I use
FOLFOX in patients with Stage III disease, except in those
who refuse the combination, cannot take a neurotoxic drug
or are too old for such a combination. Those patients who
don’t receive adjuvant FOLFOX receive single-agent
capecitabine. The next question becomes: Can we combine capecitabine
with oxaliplatin? Adjuvant CAPOX is still experimental, and
it should be used as part of a clinical trial. We still have
to wait for the head-tohead comparison with FOLFOX.
For patients with Stage II disease, it becomes more interesting.
You may still want to use adjuvant FOLFOX, although in the
original European trial, less benefit was seen in patients
with Stage II disease (Andre 2004). In general, our nonprotocol
approach in patients with Stage II disease is to use single-agent
capecitabine. We’re increasingly administering adjuvant
chemotherapy in patients with Stage II disease, and capecitabine
is our drug of choice.
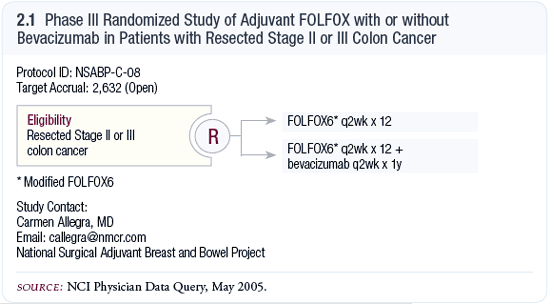
NSABP-C-08: Phase III randomized trial
of adjuvant FOLFOX with or without bevacizumab in patients
with resected Stage II or III colon cancer
The specific question being asked by NSABP-C-08 (2.1) relates
to the use of bevacizumab. The duration of therapy with bevacizumab
is also of interest in this study because it continues after
adjuvant chemotherapy for another six months. The rationale
for that remains to be seen, because we don’t know
whether we should use it for six months, 12 months or 24
months. Does adjuvant bevacizumab have a benefit beyond that
associated with adjuvant chemotherapy? Does bevacizumab alone
have any activity?
We also have to evaluate the toxicity associated with this
regimen because of what we’ve seen with bevacizumab.
NSABP-C-08 is a good trial because the best use of bevacizumab
might be early in the natural history of the disease. This
may be the way to go, but one of the concerns with the regimen
is, obviously, toxicity. We’ll need to see what happens.
NSABP-R-04: Phase III randomized trial
in patients with rectal cancer of neoadjuvant chemoradiation
therapy with capecitabine versus intravenous 5-FU/leucovorin
with or without oxaliplatin
I have mixed thoughts with respect to the first randomization
in NSABP-R-04 because I already use capecitabine with radiation
therapy. We started using this at our institution several
years ago when there weren’t any protocols available.
We reviewed and published our experience confirming the safety
of this approach (Vaishampayan 2002); therefore, we have
been using capecitabine routinely in these patients off protocol.
I am interested in the second randomization in the trial
using oxaliplatin. I have started using that in combination
with radiation therapy in some, but not all, patients. For
example, I have used oxaliplatin in healthier patients with
a better performance status and in patients with whom I have
special concerns about not being able to preserve the sphincter,
where I want to obtain a maximum pathologic response.
Clinical approach to the management of
patients with metastatic disease
At our institution, we evaluated the combination of capecitabine
and oxaliplatin (CAPOX). At this time, our front-line nonprotocol
treatment approach includes bevacizumab and CAPOX. Granted,
no Phase III trial data are available comparing CAPOX to
FOLFOX.
However, in the metastatic disease setting, taking into
account the convenience for patients of receiving an oral
agent instead of continuous infusion 5-FU, we feel that CAPOX
would be better than FOLFOX. I probably would not make the
same comment for adjuvant therapy. But in the metastatic
disease setting, my approach would be bevacizumab plus CAPOX.
For patients with disease that has progressed on an oxaliplatin-based
treatment, we move to an irinotecan-based therapy. The question
becomes: Do we use irinotecan as a single agent or in combination
with a fluoropyrimidine (eg, capecitabine or 5-FU/leucovorin)?
The third- or fourth-line options would be any of the above
with or without cetuximab.
Select publications
 |
Dr Philip is a Professor of Hematology
and Oncology at Karmanos Cancer Institute, Wayne
State University in Detroit, Michigan. |
|

