You are here: Home: CCU 4 | 2005: Robert
B Diasio, MD
| Robert B Diasio,
MD |
EDITED
COMMENTS |
 Clinical trials of adjuvant therapy for
colorectal cancer Clinical trials of adjuvant therapy for
colorectal cancer
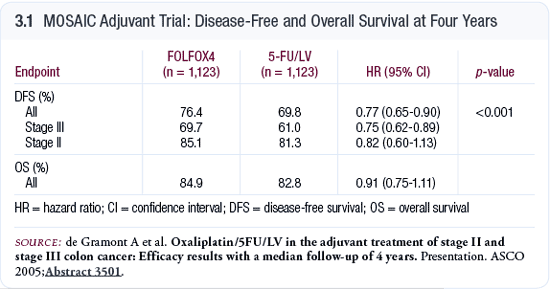
The MOSAIC trial
The MOSAIC trial (de Gramont 2003) randomly assigned Stage
II and III patients to the FOLFOX4 regimen with oxaliplatin
as opposed to the typical infusional 5-FU/ leucovorin regimen
used in France.
This multinational study demonstrated almost a 25 percent
reduction in the rate of metastasis in patients with Stage
III disease who received the FOLFOX regimen.
We now have longer follow-up data from the MOSAIC trial
(Andre 2004; de Gramont 2005; [3.1]). While the data for
Stage II disease are still not statistically significant,
the data in patients with Stage III are even better, and
we’re beginning to approach the parameters to evaluate
overall survival data. A definite improvement with FOLFOX
was shown in the MOSAIC trial, and based on these data, the
FDA approved this regimen as adjuvant therapy in Stage III
disease.
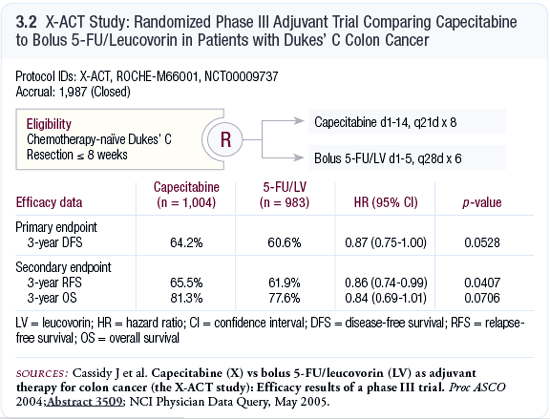
The X-ACT trial
The other adjuvant study we should note is the X-ACT trial,
which compared capecitabine 1,250 mg/m2 bid versus the Mayo
Clinic 5-FU/leucovorin regimen in patients with resected
Dukes’ C colon cancer. This is an intriguing study
because it addresses the clinical issue of using oral 5-FU
for patients who are in relatively good health, have a good
performance status and who oftentimes can continue to work.
For them, the option of using an oral agent rather than intravenous
therapy is very attractive.
Overall survival cannot be evaluated at this point, but
disease-free survival was superior for patients on the capecitabine
arm (Cassidy 2004; [3.2]). The p-value was approximately
0.05, bordering significance, as did the hazard ratio with
the confidence intervals approaching the 1.00 mark but not
crossing it. That’s important because it suggests that
this is a statistically significant effect.
ASCO paper on adjuvant therapy in Stage
II disease
Typically, patients with no evidence of lymph node involvement,
no matter how deeply the tumor appears to extend, do not
receive chemotherapy for Stage II disease. However, increasing
data suggest that some patients with penetration of the intestinal
wall, who would not have been treated in the past, may benefit
from chemotherapy.
The ASCO committee published an aggressive position paper
stating that perhaps these patients should be offered adjuvant
therapy (Benson 2004). While we don’t have any convincing
objective data to validate the use of adjuvant therapy in
Stage II disease, subsets within that population may benefit.
The ultimate proof of the benefit in such patients will come
from ongoing adjuvant studies.
One reason it may be difficult to demonstrate a benefit
from adjuvant therapy in Stage II disease is that fewer events
occur. However, the MOSAIC trial and some of the earlier
Intergroup studies have suggested certain patients can benefit
from chemotherapy.
I believe we’re at the point now that with consideration
of the tumor’s histologic characterization, the localization,
whether it’s in the right or left side of the colon,
the occurrence of methylation, microsatellite instability,
TS, p53 and various other new markers being identified that
we may be able to identify subsets that will benefit from
adjuvant therapy.
Capecitabine/oxaliplatin in the adjuvant
setting
The MOSAIC trial was reported in 2003 and led to the FDA
approval of FOLFOX4 for adjuvant therapy in Stage III disease.
The X-ACT trial data were reported in 2004, and we expect
capecitabine will be approved in this setting also. At this
time, FOLFOX appears to be superior, and that might limit
the overall use of capecitabine.
However, down the road, with the availability of oxaliplatin
in the practice setting, practitioners may begin to do what
they did in advanced disease — use CAPOX. The CAPOX
regimen is very appealing to a number of oncologists and
patients, but we have no data on that combination in the
adjuvant setting.
Adjuvant therapy in the nonprotocol setting
While some clinicians are still using 5-FU/leucovorin in
the adjuvant setting, now that FOLFOX has been approved,
I believe we’ll see a change in treatment patterns
in the adjuvant setting just as we’ve seen in advanced
disease. Whether adjuvant 5-FU/leucovorin without oxaliplatin
is justifiable depends on the individual patient.
For example, for an elderly patient unable to take oxaliplatin,
5-FU/leucovorin could be considered. That’s also a
situation in which capecitabine might have a role, based
on the X-ACT study, which provides strong evidence that capecitabine
is better than 5-FU/leucovorin in the adjuvant setting.
However, I believe that for the majority of patients who
have reasonable performance status and are in excellent health,
it’s preferable to use adjuvant FOLFOX.
Capecitabine as adjuvant therapy for patients with Stage
II disease is appealing because it’s an oral agent,
and given the ASCO position paper on treating Stage II disease,
I foresee increased use of this. We just don’t have
the clinical data at this point.
Select publications
 |
Dr Diasio is a Professor of Medicine
(Hematology/Oncology) and Pharmacology/Toxicology
and Genetics, Associate Director for Basic Sciences
at UAB Comprehensive Cancer Center, Chairman of
the Department of Pharmacology and Toxicology and
the Newman H Waters Professor and Director, Division
of Clinical Pharmacology at the University of Alabama
Birmingham, in Birmingham, Alabama. |
|

