You are here: Home: CCU 4 | 2005: Bruce
J Giantonio, MD
| Bruce J Giantonio,
MD |
EDITED
COMMENTS |
 ECOG-E3200: FOLFOX4 with bevacizumab versus
FOLFOX4 versus bevacizumab in patients with previously treated
advanced colorectal cancer ECOG-E3200: FOLFOX4 with bevacizumab versus
FOLFOX4 versus bevacizumab in patients with previously treated
advanced colorectal cancer
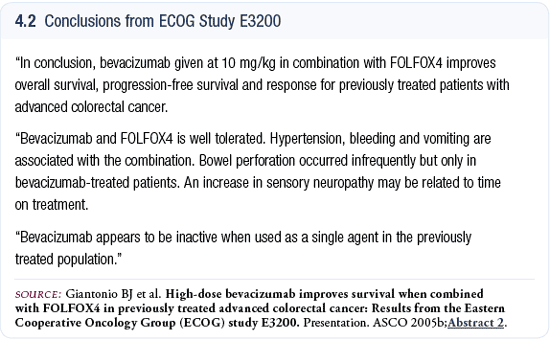
Safety
We did not observe any unexpected toxicities with the use
of bevacizumab. One of the key things to keep in mind about
ECOG-E3200 is that we used a higher dose of bevacizumab (10
mg/kg every two weeks) than has been reported in any of the
colorectal cancer studies, except for that first trial by
Dr Kabbinavar (Kabbinavar 2003). ECOG-E3200 demonstrated
statistically significant increases in nausea, vomiting and
neuropathy in the bevacizumab arm (Giantonio 2005b), which
were probably due to these patients staying on treatment
longer because of the addition of bevacizumab.
I don’t believe bevacizumab is enhancing these side
effects; rather, it’s just allowing the patients to
stay on treatment longer. We observed about a 15 percent
incidence of Grade III neuropathy in the patients treated
with FOLFOX4 and bevacizumab compared to nine percent in
the patients treated with FOLFOX4 alone (Giantonio 2005b).
We observed bowel perforations in ECOG-E3200 similar to
what was reported by Dr Hurwitz (Hurwitz 2004). We saw three
cases in the patients treated with FOLFOX4 plus bevacizumab,
three cases in the patients treated with bevacizumab alone
and no cases in the patients treated with FOLFOX4 alone.
Four of our six cases of perforation occurred within the
first cycle of therapy, which is different from Dr Hurwitz’s
experience. In his IFL plus bevacizumab study, there was
no association with time on treatment and the development
of the perforation.
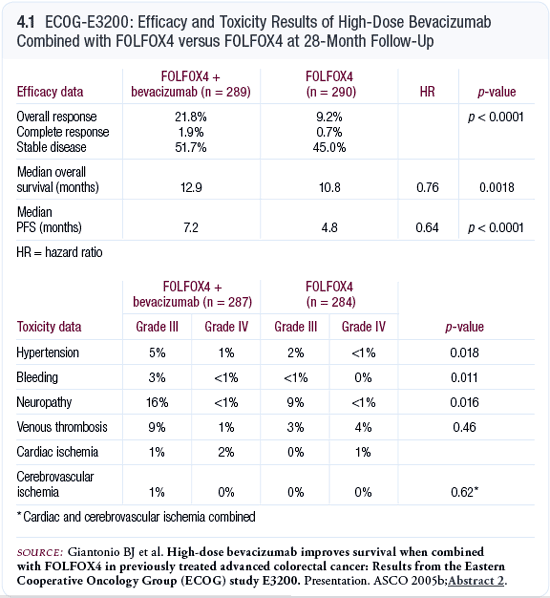
Efficacy
In October 2004, an interim analysis was conducted and
presented to the ECOG Data Monitoring Committee. Based on
their review, they recommended the release of the study data.
At the Data Monitoring Committee’s meeting, a statistically
significant (p = 0.0024) improvement in median overall
survival — the primary endpoint of the trial — was
reported. The patients who received FOLFOX4 plus bevacizumab
had a median overall survival of 12.5 months compared to
10.7 months for those treated with FOLFOX4 alone. The hazard
ratio was 0.74; patients treated with bevacizumab in combination
with FOLFOX had a 26 percent reduction in the risk of death
(Giantonio 2005a; [4.1, 4.2]).*
It was recognized that these data were important because
FOLFOX has evolved as front-line therapy for patients with
metastatic colorectal cancer in the United States. Even though
ECOG-E3200 is a study of second-line therapy, it makes many
of us more comfortable in extrapolating these data to the
front-line setting.
Clinical implications of ECOG-E3200
I think ECOG-E3200 adds very strongly to the existing data
that bevacizumab in combination with FOLFOX, as first-line
therapy, should further improve median overall survival.
Given what we can accomplish with chemotherapy alone using
FOLFOX and FOLFIRI, as demonstrated by Dr Tournigand (Tournigand
2004), conceivably, we can anticipate starting to move the
median overall survival to two or more years. That is remarkable
when just four years ago the median overall survival was
about 12 months for the control arm of 5-FU/leucovorin in
the IFL study (Saltz 2000).
In patients with metastatic disease, I generally start
with FOLFOX plus bevacizumab. When they progress, I switch
to FOLFIRI, and based on their insurance, I keep them on
bevacizumab. When using FOLFIRI plus bevacizumab, I had been
using a dose of 5 mg/kg; however, based on the results from
ECOG-E3200, I’ll probably try to increase the dose
to 10 mg/kg if I can obtain approval from the patient’s
insurance company.
In terms of the management of some of the concerning side
effects, particularly bowel perforation, I think it shouldn’t
deter clinicians from using bevacizumab. All these perforations
presented with abdominal pain. I think we just have to be
a bit more vigilant in our evaluation. With prompt intervention,
the bowel perforation can be effectively managed.
Regimens of bevacizumab in combination
with oxaliplatin or irinotecan
The magnitude of benefit for FOLFOX4 with bevacizumab in
ECOG-E3200 was about two months (Giantonio 2005a, 2005b),
and the magnitude of benefit for IFL with bevacizumab, as
reported by Dr Hurwitz, was a little more than four months
(Hurwitz 2004). The important difference was that IFL was
being used as first-line therapy.
A randomized Phase III study being conducted in Europe
will ask a survival question for the addition of bevacizumab
to FOLFOX in the first-line setting. If the magnitude of
benefit is the same, I think that will add to the data set
indicating that it is important for bevacizumab to be administered
with chemotherapy.
One of the intriguing hypotheses, at least in colorectal
cancer, is that the benefit seen with the addition of bevacizumab
may be independent of the specific chemotherapy used. A number
of mechanisms have been proposed to help explain the benefit
associated with bevacizumab. One is that there may be improved
chemotherapy delivery into the tumor, resulting in a higher
tumor kill. There’s some merit to that, and emerging
clinical data support it.
Dr Willett has looked at the changes in microvascular density
and interstitial pressures in tumors from patients who have
received bevacizumab. The patients underwent colonoscopy
to have the measurements performed on the tumor, received
a single dose of bevacizumab and 12 days later had a second
colonoscopy to have those measurements repeated (Willett
2004a, 2004b).
In his study, we saw both a reduction in microvascular
density and an improvement in interstitial pressures, which
enhances the flow through the tumor and potentially allows
greater drug delivery into the tumor.
Select publications
* Data discussed in the interview reflect data presented
at ASCO GI 2005. Updated data presented at ASCO 2005 are
shown in 4.1.
 |
Dr Giantonio is an Assistant Professor
of Medicine at the Abramson Cancer Center of the
University of Pennsylvania in Philadelphia, Pennsylvania. |
|

