
| Tracks 1-9 |
| Track 1 |
Preoperative therapy for patients undergoing potentially curative resection of hepatic metastases |
| Track 2 |
Chemotherapy with bevacizumab and an anti-EGFR antibody or cetuximab for potentially resectable metastatic disease |
| Track 3 |
Therapeutic approach for patients
with “borderline resectable”
metastatic disease |
| Track 4 |
Clinical trials evaluating biologic doublets in the metastatic setting |
| Track 5 |
Potential impact of converting unresectable hepatic metastases to resectable disease |
|
| Track 6 |
Novel biologic strategies and agents in colorectal cancer |
| Track 7 |
Tumor location and vascularity and the resectability of hepatic metastases |
| Track 8 |
Use of FOLFOX with either
bevacizumab or cetuximab for
potentially resectable metastatic
disease |
| Track 9 |
Therapeutic options for patients
with peritoneal metastases and
liver disease |
|
|
Tracks 1-2
 DR LOVE:
DR LOVE: Howard, do you recommend preoperative chemotherapy for
patients with potentially resectable hepatic metastases?
 DR HOCHSTER: If a patient has a clearly resectable liver lesion or two, we
have a discussion with the patient and the surgeon as to whether it makes
more sense to do surgery first or to administer induction chemotherapy and then go for surgery.
DR HOCHSTER: If a patient has a clearly resectable liver lesion or two, we
have a discussion with the patient and the surgeon as to whether it makes
more sense to do surgery first or to administer induction chemotherapy and then go for surgery.
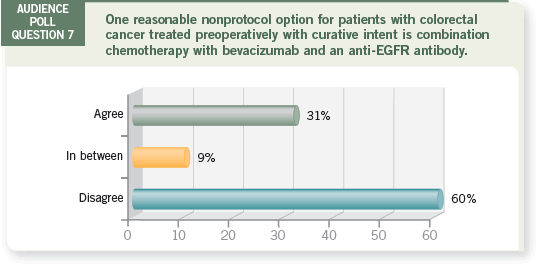
I believe administering chemotherapy first is an option, and while we don’t
have data showing that induction chemotherapy improves survival, we hope
the EORTC-40983 trial will show that eventually.
In this Phase III trial, patients with potentially resectable liver metastases were
randomly assigned to receive three months of FOLFOX before and three
months of FOLFOX after surgery or to undergo surgery without chemotherapy
(Gruenberger 2006b; [7.1]).
If the trial demonstrates a survival benefit for chemotherapy, then we will
know to treat these patients with chemotherapy up front. However, you have
to bear in mind that the systemic therapy in the trial is FOLFOX alone, not FOLFOX with bevacizumab.
 DR LOVE: How do you treat the patient who has either unresectable liver
disease or one or two metastases in the lung in addition to the liver metastases?
DR LOVE: How do you treat the patient who has either unresectable liver
disease or one or two metastases in the lung in addition to the liver metastases?
 DR HOCHSTER: In those cases, I prefer FOLFOX with bevacizumab because
that combination has the highest response rate. The first-line data from the
somewhat abbreviated SWOG study show that cetuximab also improves the response rate when combined with chemotherapy, so we have two antibodies
that increase the response rate over chemotherapy alone.
DR HOCHSTER: In those cases, I prefer FOLFOX with bevacizumab because
that combination has the highest response rate. The first-line data from the
somewhat abbreviated SWOG study show that cetuximab also improves the response rate when combined with chemotherapy, so we have two antibodies
that increase the response rate over chemotherapy alone.
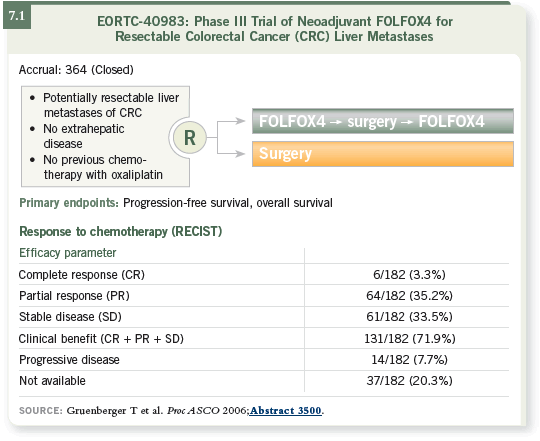
The CALGB-C80203 study, presented by Alan Venook at ASCO 2006,
was supposed to accrue 2,200 patients but was stopped early after enrolling
approximately 280. The design was a two-by-two randomization of FOLFOX
versus FOLFIRI with or without cetuximab. The addition of cetuximab
showed approximately a 10 to 20 percent increase in the response rate for both
arms, so this study also suggests that the addition of cetuximab to first-line
chemotherapy improves the response rate (Venook 2006; [7.2]).
Data from the large European study, the CRYSTAL trial, should also be
available soon. This trial compares FOLFIRI with or without cetuximab for
first-line therapy. According to the recent press release, cetuximab did increase
progression-free survival, which was the primary endpoint.
Track 4
 DR LOVE:
DR LOVE: Can you review the BOND-2 trial?
 DR HOCHSTER: This was a Phase II, NCI consortium trial that we participated in with Leonard Saltz at Memorial. The trial evaluated patients whose
disease had progressed on an irinotecan regimen but who had not received
bevacizumab previously. Patients who received both bevacizumab and cetuximab
had approximately a 20 percent response rate, as compared to 11 percent
among patients who received cetuximab only.
DR HOCHSTER: This was a Phase II, NCI consortium trial that we participated in with Leonard Saltz at Memorial. The trial evaluated patients whose
disease had progressed on an irinotecan regimen but who had not received
bevacizumab previously. Patients who received both bevacizumab and cetuximab
had approximately a 20 percent response rate, as compared to 11 percent
among patients who received cetuximab only.
The toxicities were as expected from either antibody, with no unexpected or
synergistic toxicities (Saltz 2005). They saw vascular side effects and perforations
from the bevacizumab and skin toxicities from the cetuximab.
 DR LOVE: What other ongoing studies are evaluating the double antibody
approach?
DR LOVE: What other ongoing studies are evaluating the double antibody
approach?
 DR HOCHSTER: Two studies are examining this strategy in the front-line
setting. The PACCE study is a first-line study of FOLFOX or FOLFIRI
with bevacizumab in one arm versus bevacizumab and panitumumab in the
second arm. It has already completed accrual, and data should be available in
the next year.
DR HOCHSTER: Two studies are examining this strategy in the front-line
setting. The PACCE study is a first-line study of FOLFOX or FOLFIRI
with bevacizumab in one arm versus bevacizumab and panitumumab in the
second arm. It has already completed accrual, and data should be available in
the next year.
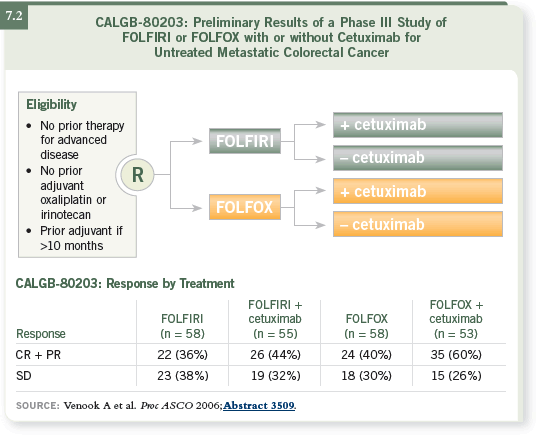
The other study is the CALGB Intergroup study, C80405, which is a three-arm
study powered for survival, so it is a much bigger study. The physician
selects either FOLFOX or FOLFIRI, and then patients are randomly assigned
to bevacizumab alone, cetuximab alone or the combination of the two (7.3).
That’s an excellent study, which, if completed, will show the merits of using an anti-VEGF antibody, an anti-EGF antibody or the combination of the two.
That should give us some clear data.
Track 8
 DR LOVE:
DR LOVE: Axel, what do you consider a reasonable approach to treating
patients with potentially resectable metastatic disease in practice?
 DR GROTHEY: I believe what we are seeking in the neoadjuvant setting is
response rate, more than delaying of tumor progression, but we don’t want
to get to the point where we can no longer see the metastases. I have heard
the statement, “The medical oncologist’s dream is a surgeon’s nightmare.” We
know that a complete response in a liver metastasis is not a complete response
by the pathology criteria.
DR GROTHEY: I believe what we are seeking in the neoadjuvant setting is
response rate, more than delaying of tumor progression, but we don’t want
to get to the point where we can no longer see the metastases. I have heard
the statement, “The medical oncologist’s dream is a surgeon’s nightmare.” We
know that a complete response in a liver metastasis is not a complete response
by the pathology criteria.
I can easily see the rationale for using EGF receptor antibodies based on
Alan’s data and other data (Venook 2006). A consistent response rate benefit
occurs when we add cetuximab. However, the data are limited, and we’re still
waiting on the Phase III first-line trial results with cetuximab.
Whether we should also add bevacizumab is a different question. For now, I
believe that in first-line, neoadjuvant therapy, response rate is our surrogate
marker for resectability, but how we get there is an area of discussion.
 DR LOVE: What do you currently use in clinical practice?
DR LOVE: What do you currently use in clinical practice?
 DR GROTHEY: Off study, I’ve used FOLFOX/bevacizumab, and I’ve used
FOLFOX/cetuximab.
DR GROTHEY: Off study, I’ve used FOLFOX/bevacizumab, and I’ve used
FOLFOX/cetuximab.
 DR LOVE: Alan, how do you feel about double biologic therapy in this
setting?
DR LOVE: Alan, how do you feel about double biologic therapy in this
setting?
 DR VENOOK: One of the endpoints of the Phase III CALGB-C80405 trial,
the study of cetuximab and/or bevacizumab with FOLFOX or FOLFIRI in
patients with previously untreated metastatic colorectal cancer, is to analyze
the number of patients who go to surgery. However, off study, I would not use
double biologics, largely due to the insurance issues.
DR VENOOK: One of the endpoints of the Phase III CALGB-C80405 trial,
the study of cetuximab and/or bevacizumab with FOLFOX or FOLFIRI in
patients with previously untreated metastatic colorectal cancer, is to analyze
the number of patients who go to surgery. However, off study, I would not use
double biologics, largely due to the insurance issues.
 DR LOVE: How do you feel about using FOLFOXIRI in this setting?
DR LOVE: How do you feel about using FOLFOXIRI in this setting?
 DR VENOOK: Data in the literature are conflicting regarding FOLFOXIRI.
A paper from the Hellenic Oncology Society in Greece in the British Journal
of Cancer showed more toxicity and no benefit with FOLFOXIRI when
compared to FOLFIRI (Souglakos 2006).
DR VENOOK: Data in the literature are conflicting regarding FOLFOXIRI.
A paper from the Hellenic Oncology Society in Greece in the British Journal
of Cancer showed more toxicity and no benefit with FOLFOXIRI when
compared to FOLFIRI (Souglakos 2006).
Generally, our approach is to treat these patients with four cycles of
FOLFOX/bevacizumab. If at that point the tumor is deemed resectable, we
stop the bevacizumab, administer another dose of FOLFOX and then resect
six weeks after the last dose of bevacizumab.
We have to be careful not to offer too much treatment to these patients preoperatively, or we may eradicate the disease and the surgeon doesn’t know where
to go, believe it or not. Also, it may result in a fatty liver, and the experienced
hepatic surgeon can tell you that the liver is like mush in some of these
patients who have been too heavily pretreated.
Select Publications

