
| Tracks 1-6 |
| Track 1 |
Planned “holidays” from oxaliplatin or irinotecan during treatment with FOLFOX or FOLFIRI |
| Track 2 |
Incorporation of biologic therapies
in chemotherapy-free intervals
and maintenance strategies |
| Track 3 |
Complete treatment-free intervals versus maintenance therapy and the role of biologic therapies |
|
| Track 4 |
Considerations in defining the
end of treatment holidays |
| Track 5 |
Peripheral neuropathy and the decision to discontinue or reintroduce oxaliplatin |
| Track 6 |
Failure to utilize maintenance therapy: Impact on progression-free survival in XELOX-1/NO16966 |
|
|
Track 1
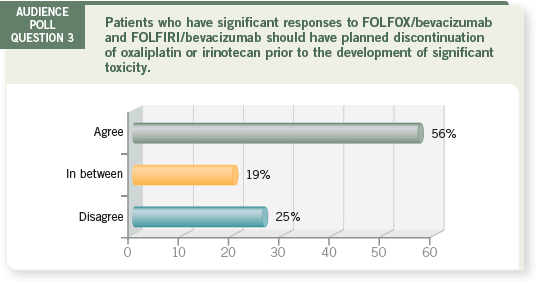
 DR LOVE:
DR LOVE: Axel, what are your thoughts about this poll question?
 DR GROTHEY: I was happy about the audience answers because I have been
trying to educate physicians to stop treatment before patients develop toxicities.
DR GROTHEY: I was happy about the audience answers because I have been
trying to educate physicians to stop treatment before patients develop toxicities.
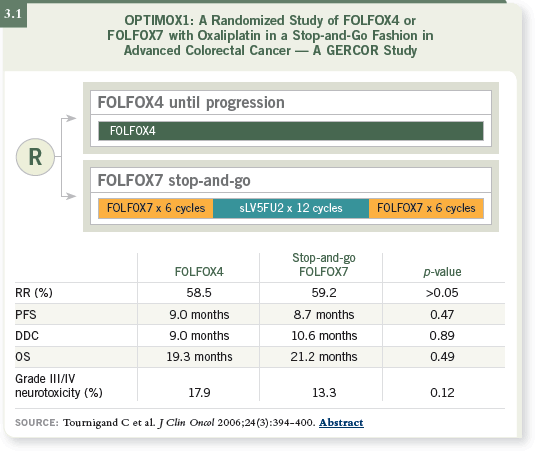
For an oxaliplatin-based regimen, this approach has evolved, and it’s already the standard approach based on the Phase III data from the OPTIMOX1 trial,
which demonstrated we can safely discontinue oxaliplatin without compromising
efficacy (Tournigand 2006; [3.1]).
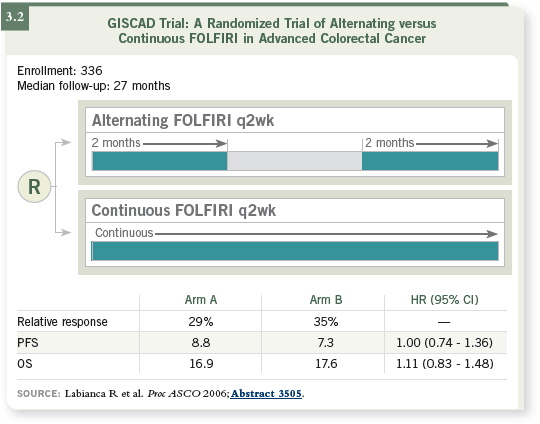
For irinotecan, we have limited information based on the Italian GISCAD
trial presented at ASCO 2006. Discontinuation of irinotecan-based therapy
— in an on-and-off schedule of two months on FOLFIRI followed by two
months off FOLFIRI (a complete chemotherapy break) — did not influence
progression-free survival or toxicity (Labianca 2006; [3.2]), which was interesting.
The data are more solid for an oxaliplatin-based regimen, which is what we
use more often as first-line therapy in the United States. Because oxaliplatin
is associated with cumulative toxicity, it makes sense to “OPTIMOXize”
FOLFOX, meaning to stop oxaliplatin after a certain number of cycles.
At the 2007 ASCO GI Symposium, Aimery de Gramont discussed the history
of the OPTIMOX trials and updated the results from the OPTIMOX2 trial.
The problem Aimery faced when he developed the FOLFOX regimen was
that more patients stopped oxaliplatin-based therapy because of toxicity than
because of disease progression (Green 2005).
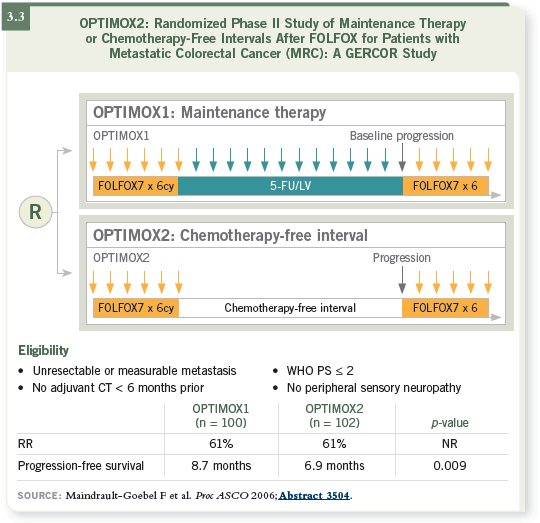
So the OPTIMOX1 trial in patients with metastatic colorectal cancer
compared the continuation of FOLFOX4 until disease progression or toxicity
to a stop-and-go strategy for oxaliplatin, which used induction therapy with
six cycles of FOLFOX7 followed by six months of maintenance therapy with
5-FU/leucovorin and planned reintroduction of FOLFOX7. He demonstrated
that when you stop oxaliplatin for six months, this does not compromise
overall outcome in terms of the response rate, progression-free survival,
duration of disease control and overall survival (Tournigand 2006; [3.1]).
The OPTIMOX2 trial randomly assigned patients with metastatic colorectal
cancer to an OPTIMOX1-like treatment arm of induction, maintenance,
reintroduction or a treatment arm consisting of induction, a complete chemotherapy-free interval and reintroduction. It was a Phase II trial comparing
maintenance therapy to a complete break of any tumor-directed therapy
(Maindrault-Goebel 2006; [3.3]).
The response rates were the same for the patients treated with maintenance
therapy and those treated with a chemotherapy-free interval because they
received the same induction therapy regimen. There is no question, and
response occurs early (Maindrault-Goebel 2006).
It’s clear that when you receive some tumor-targeted therapy (ie, chemotherapy that can inhibit tumor growth), progression-free survival is significantly
longer than if you stop therapy completely (Maindrault-Goebel 2006;
[3.3]).
If progression-free survival is your primary endpoint, you must be sure
patients receive their therapy and only discontinue the treatment components
that create toxicity.
 DR LOVE: How is this approach being integrated into the current randomized
trials in the metastatic setting?
DR LOVE: How is this approach being integrated into the current randomized
trials in the metastatic setting?
 DR GROTHEY: We still have a hard time adopting this stop-and-go approach
in our ongoing Phase III trials that use an oxaliplatin-based regimen up front.
At some point patients will discontinue treatment not for progression but for
toxicity, which then affects overall outcome if not dealt with in the right way.
Right now, the ongoing Intergroup trial is being amended to include a more
or less OPTIMOX-like approach as the standard.
DR GROTHEY: We still have a hard time adopting this stop-and-go approach
in our ongoing Phase III trials that use an oxaliplatin-based regimen up front.
At some point patients will discontinue treatment not for progression but for
toxicity, which then affects overall outcome if not dealt with in the right way.
Right now, the ongoing Intergroup trial is being amended to include a more
or less OPTIMOX-like approach as the standard.
Track 2
 DR LOVE:
DR LOVE: What do we know about incorporating biologics into this type
of strategy?
 DR GROTHEY: We have limited data about how biologics would affect
this concept of maintenance therapy or chemotherapy-free intervals. We’re
currently conducting a trial led by the Mayo Clinic, which is evaluating a
FOLFOX regimen with bevacizumab in a stop-and-go design.
DR GROTHEY: We have limited data about how biologics would affect
this concept of maintenance therapy or chemotherapy-free intervals. We’re
currently conducting a trial led by the Mayo Clinic, which is evaluating a
FOLFOX regimen with bevacizumab in a stop-and-go design.
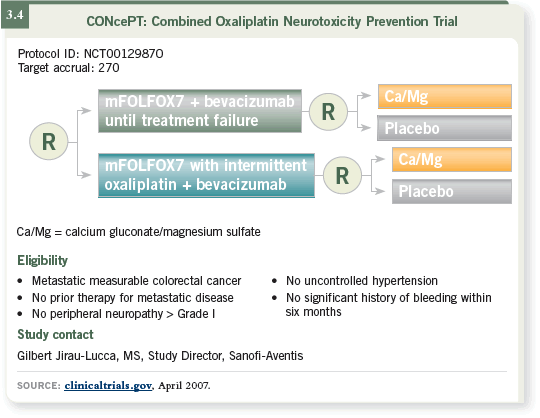
In this trial, patients receive FOLFOX and bevacizumab for four months or
eight cycles (3.4). Then we apply a planned discontinuation of oxaliplatin
while 5-FU/bevacizumab is continued for four months, followed by the
reintroduction of oxaliplatin.
This trial has accrued more than half of the target accrual goal, so we should
have some data next year to determine whether continuing biologics as an
integral component of maintenance therapy allows us to deliver more treatment
and delay tumor progression without affecting toxicity.
Currently, various trials use biologic agents in the maintenance phase to delay
tumor progression. The idea is, you might induce a response by using conventional chemotherapy in combination with one or two biologic agents and
maintain the response by using biologic agents to allow for a long-term delay
in progression and limit toxicity.
Tracks 3-4
 DR LOVE:
DR LOVE: Neal, in practice do you use treatment-free intervals or prefer
some type of maintenance therapy?
 DR MEROPOL: In general, I provide complete treatment-free holidays. It is a
tremendous added benefit for the patient not to have to come in to the office.
While we have no secure data addressing the survival impact of a complete
holiday, in the absence of data to the contrary and with the data from the
OPTIMOX1, OPTIMOX2 and GISCAD studies suggesting no detriment, at
least in short-term outcomes, I’m pretty comfortable discussing with a patient
the possibility of a complete holiday from treatment.
DR MEROPOL: In general, I provide complete treatment-free holidays. It is a
tremendous added benefit for the patient not to have to come in to the office.
While we have no secure data addressing the survival impact of a complete
holiday, in the absence of data to the contrary and with the data from the
OPTIMOX1, OPTIMOX2 and GISCAD studies suggesting no detriment, at
least in short-term outcomes, I’m pretty comfortable discussing with a patient
the possibility of a complete holiday from treatment.
 DR HALLER: A question I have for the panel members is, what defines the end
of the holiday, assuming you don’t want to wait until the patient is symptomatic?
DR HALLER: A question I have for the panel members is, what defines the end
of the holiday, assuming you don’t want to wait until the patient is symptomatic?
 DR GOLDBERG: So many different considerations are relevant — including
the patient’s psychology. For some patients, the thought that they have cancer
and are not actively on treatment is so detrimental to their quality of life that
they’d rather put up with the toxicities and the office visits.
DR GOLDBERG: So many different considerations are relevant — including
the patient’s psychology. For some patients, the thought that they have cancer
and are not actively on treatment is so detrimental to their quality of life that
they’d rather put up with the toxicities and the office visits.
 DR VENOOK: I favor absolute holidays, but they are not appropriate for
everyone. Palliation means trying to improve the patient’s quality of life, and
stopping therapy can often help to do that. However, the issue is complex, and
my default position is probably to continue the chemotherapy. Also, we need a
data set on whether discontinuing bevacizumab will be a problem.
DR VENOOK: I favor absolute holidays, but they are not appropriate for
everyone. Palliation means trying to improve the patient’s quality of life, and
stopping therapy can often help to do that. However, the issue is complex, and
my default position is probably to continue the chemotherapy. Also, we need a
data set on whether discontinuing bevacizumab will be a problem.
Select Publications

