
| Tracks 1-8 |
| Track 1 |
Use of bevacizumab for patients with prior arterial events |
| Track 2 |
Potential mechanisms underlying bevacizumab-associated arterial thromboembolic events |
| Track 3 |
Surgery after treatment with bevacizumab |
| Track 4 |
Reversible posterior leukoenceph-alopathy syndrome (RPLS) and bevacizumab |
|
| Track 5 |
Dose of bevacizumab, use of
aspirin and risk of thromboembolic
events or bleeding |
| Track 6 |
Balancing risks and benefits of bevacizumab |
| Track 7 |
Perspectives on the long-term use of bevacizumab in the adjuvant and metastatic settings |
| Track 8 |
Treatment of bevacizumab-associated hypertension |
|
|
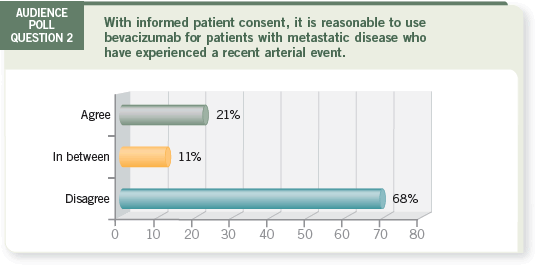
Track 1
 DR LOVE:
DR LOVE: Alan, how would you respond to the poll question — agree,
disagree or in between?
 DR VENOOK: I don’t believe a global “no” or “yes” is the right answer when
asked this question. With every treatment decision, we weigh the benefits and
risks of therapy.
DR VENOOK: I don’t believe a global “no” or “yes” is the right answer when
asked this question. With every treatment decision, we weigh the benefits and
risks of therapy.
Clearly the evidence suggests that patients who’ve had prior arterial thromboembolic
events (ATEs) are at greater risk for developing subsequent events
when treated with bevacizumab (2.1), and one should remember that the contemporary bevacizumab studies excluded patients who had experienced a
variety of ATEs within a specific time frame, usually a year.
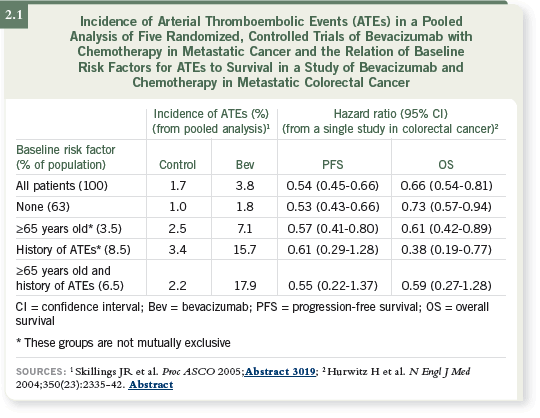
Therefore, I believe it’s prudent to think twice before treating a patient who’s
had a myocardial infarction (MI) or ATE in the past year. Still, it’s a relative
contraindication because the average patient will benefit from bevacizumab,
and if you examine the data, you’ll see that the incidence of an ATE is only
about four percent (Hedrick 2006).
When I examine this issue, I consider a couple of angles. One, what is the goal
of therapy? If you believe that an excellent response to treatment might render
a patient curable and free of disease, then you want to give that patient the
best shot, and that might be a reason to use bevacizumab even with a relative
contraindication.
On the other hand, if a patient has massive metastatic disease and the goal
is clearly palliative, you might think twice about using bevacizumab if that
patient is marginal in terms of risk factors for these events.
 DR LOVE: Alan, what about the patient on anticoagulation receiving bevacizumab?
DR LOVE: Alan, what about the patient on anticoagulation receiving bevacizumab?
 DR VENOOK: While a bit of a myth suggests that patients who are anticoagulated
can’t safely receive bevacizumab, a fair amount of data indicates that’s not the case. I see patients who do not receive bevacizumab because of a perceived
contraindication that I believe to be incorrect. A prime example is the patient
who’s been anticoagulated for a coronary stent placed five years ago. I see no
contraindication to bevacizumab when treating a patient like that.
DR VENOOK: While a bit of a myth suggests that patients who are anticoagulated
can’t safely receive bevacizumab, a fair amount of data indicates that’s not the case. I see patients who do not receive bevacizumab because of a perceived
contraindication that I believe to be incorrect. A prime example is the patient
who’s been anticoagulated for a coronary stent placed five years ago. I see no
contraindication to bevacizumab when treating a patient like that.
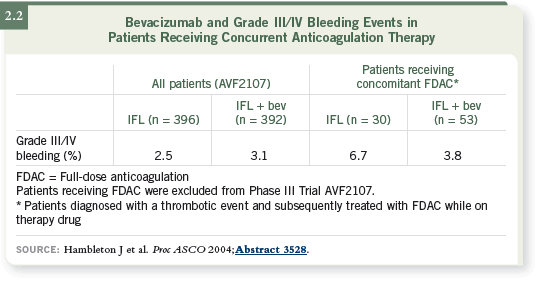
 DR LOVE: Do we know what that misperception is based on?
DR LOVE: Do we know what that misperception is based on?
 DR VENOOK: I presume that at the beginning of the studies, there was global
concern regarding clotting and bleeding. The existing data set is a retrospective
collation of results on patients who were anticoagulated, so it’s flawed by
the questions of patient selection and why they were anticoagulated.
However, Julie Hambleton has compiled data that I believe compellingly
demonstrate that if bevacizumab is otherwise indicated, the patient who’s been
anticoagulated can safely receive bevacizumab (Hambleton 2004, 2005; [2.2,
2.3]).
DR VENOOK: I presume that at the beginning of the studies, there was global
concern regarding clotting and bleeding. The existing data set is a retrospective
collation of results on patients who were anticoagulated, so it’s flawed by
the questions of patient selection and why they were anticoagulated.
However, Julie Hambleton has compiled data that I believe compellingly
demonstrate that if bevacizumab is otherwise indicated, the patient who’s been
anticoagulated can safely receive bevacizumab (Hambleton 2004, 2005; [2.2,
2.3]).
Track 4
 DR LOVE:
DR LOVE: Have you seen any cases of reversible posterior leukoencephalopathy
syndrome (RPLS)?
 DR VENOOK: I’ve never seen it — although I did have a couple of patients
with unexplained neurologic syndromes before we knew that RPLS existed,
so it’s possible that we have seen it but didn’t know.
DR VENOOK: I’ve never seen it — although I did have a couple of patients
with unexplained neurologic syndromes before we knew that RPLS existed,
so it’s possible that we have seen it but didn’t know.
It appears to be a capillary leak syndrome in patients receiving bevacizumab. It
may be related to the more severe hypertension that some patients develop on
this agent. It’s described as a diminished mentation, a seizure-like activity and
lip smacking.
I believe the real issue is distinguishing RPLS from a stroke or a bleed. We are
now more likely to perform scans on patients who have unexplained neurologic
problems. It’s possible that some of the strokes reported early on with
bevacizumab may have been this syndrome, and we didn’t work them up
adequately. Again, I’ve never seen a case, possibly because we err on the side
of stopping bevacizumab if patients are having suspicious symptoms.
Track 6
 DR LOVE:
DR LOVE: Rich, how do you feel about the use of bevacizumab in older
patients?
 DR GOLDBERG: We don’t have data to suggest that bevacizumab is tolerated
less well by older people, unless they have a history of an ATE and are over 65,
so I recommend it routinely without consideration for age, but I do consider
the patient’s arterial thrombotic history.
DR GOLDBERG: We don’t have data to suggest that bevacizumab is tolerated
less well by older people, unless they have a history of an ATE and are over 65,
so I recommend it routinely without consideration for age, but I do consider
the patient’s arterial thrombotic history.
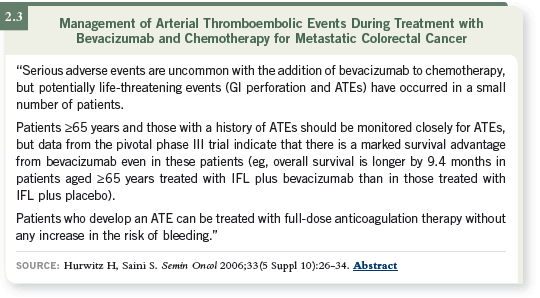
 DR HOCHSTER: In the toxicity analysis, the risk for patients over the age of 65
was increased, and the risk for patients with a recent arterial event was increased
(2.1). The risk for patients with both was increased the most, but they all had
the same survival benefit (2.1, 2.3). Part of my discussion with patients such as
these is to tell them, “Your risk of having another arterial event may be up to
10 percent, but this is also likely to prolong your life by 33 percent.”
DR HOCHSTER: In the toxicity analysis, the risk for patients over the age of 65
was increased, and the risk for patients with a recent arterial event was increased
(2.1). The risk for patients with both was increased the most, but they all had
the same survival benefit (2.1, 2.3). Part of my discussion with patients such as
these is to tell them, “Your risk of having another arterial event may be up to
10 percent, but this is also likely to prolong your life by 33 percent.”
 DR MEROPOL: For me, the scenario that sometimes plays out is the patient
for whom cure is not possible and we’re discussing prolongation of survival.
Patients may not be willing to take on the added risk of a thrombotic complication
with bevacizumab if they’re in a high-risk group in the front-line setting.
DR MEROPOL: For me, the scenario that sometimes plays out is the patient
for whom cure is not possible and we’re discussing prolongation of survival.
Patients may not be willing to take on the added risk of a thrombotic complication
with bevacizumab if they’re in a high-risk group in the front-line setting.
However, in the second- or third-line setting, when we know that bevacizumab
can also improve survival after failure of front-line therapy, the tradeoff may be different. It may be the last chance to receive bevacizumab, and they
might be more willing to take the risk as they get further along.
Track 7
 DR LOVE:
DR LOVE: Alan, what do we know about the duration of therapy with
bevacizumab and the risk of complications?
 DR VENOOK: Given that bevacizumab is being used by some as maintenance
therapy, it’s important to determine whether risks, such as for ATEs, are
increased with long-term use. This is uncharted territory. Most of the data we
have are from patients with advanced disease who have received 12 months of
bevacizumab at most.
DR VENOOK: Given that bevacizumab is being used by some as maintenance
therapy, it’s important to determine whether risks, such as for ATEs, are
increased with long-term use. This is uncharted territory. Most of the data we
have are from patients with advanced disease who have received 12 months of
bevacizumab at most.
I believe one of the important endpoints of the NSABP-C-08 trial will be
whether there are any long-term consequences from using bevacizumab for a
year in patients with Stage II or Stage III colon cancer. Meanwhile, we should
be cautious about assuming that long-term use of bevacizumab is beneficial for
patients and should be continued indefinitely.
 DR GROTHEY: We need to start a registry of patients coming off NSABP-C-08 and other trials and follow them long term in order to capture those events
and determine the risks of long-term therapy.
DR GROTHEY: We need to start a registry of patients coming off NSABP-C-08 and other trials and follow them long term in order to capture those events
and determine the risks of long-term therapy.
Select Publications

