
| Tracks 1-6 |
| Track 1 |
Incidence and treatment of patients presenting with synchronous primary and metastatic colon cancer |
| Track 2 |
NSABP-C-10: FOLFOX6 with bevacizumab for patients with unresectable Stage IV colon cancer and a synchronous asymptomatic primary tumor |
| Track 3 |
Clinical endpoints in NSABP-C-10: Events related to the intact primary tumor |
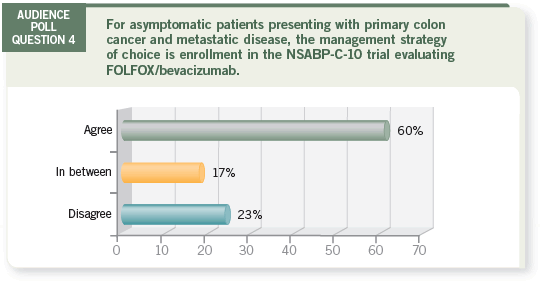
|
| Track 4 |
Treatment of patients with an intact primary tumor and synchronous metastatic disease |
| Track 5 |
Treatment approach for patients with rectal cancer and simultaneous metastatic disease |
| Track 6 |
Considerations in the addition of cetuximab to first-line chemotherapy |
|
|
Track 1
 DR LOVE:
DR LOVE: Norm, can you review the background to the NSABP-C-10
trial?
 DR WOLMARK: As surgeons, we have all been taught that the appropriate
treatment when patients present with a simultaneous primary colonic tumor
and metastatic disease is to resect the primary lesion. I have actively participated
in this practice and taught medical students and residents that if you
leave the primary tumor, then you will end up with inordinate rates of obstruction, perforation and bleeding, and you will pay a greater price later
than you would by performing the operation sooner.
DR WOLMARK: As surgeons, we have all been taught that the appropriate
treatment when patients present with a simultaneous primary colonic tumor
and metastatic disease is to resect the primary lesion. I have actively participated
in this practice and taught medical students and residents that if you
leave the primary tumor, then you will end up with inordinate rates of obstruction, perforation and bleeding, and you will pay a greater price later
than you would by performing the operation sooner.
Of course, this was a carryover from an era when therapy for metastatic
disease didn’t have the same benefits as it does currently. We wanted to
know the magnitude of the issue and whether it really is a problem today to
leave the primary intact, and we hope to answer those questions with the
NSABP-C-10 trial.
Tracks 2-3
 DR LOVE:
DR LOVE: What are the design and eligibility for the NSABP-C-10 trial?
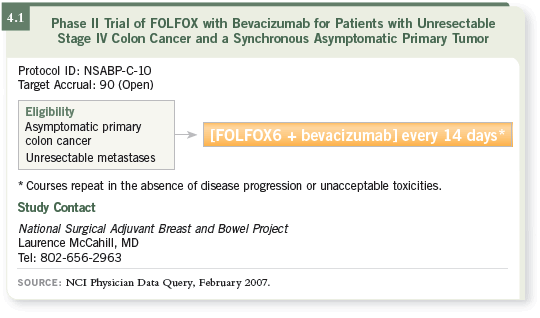
 DR WOLMARK: This is a Phase II trial evaluating FOLFOX6 with bevacizumab
in patients who present with untreated primary colon cancer and
concomitant metastatic disease not considered surgically resectable for cure
(4.1). Patients do not undergo surgery unless down the road an obstruction or
perforation makes it necessary for their safety. Patients with liver metastases
amenable to hepatic resection or resection and ablation to render them “disease
free” are ineligible for this study.
DR WOLMARK: This is a Phase II trial evaluating FOLFOX6 with bevacizumab
in patients who present with untreated primary colon cancer and
concomitant metastatic disease not considered surgically resectable for cure
(4.1). Patients do not undergo surgery unless down the road an obstruction or
perforation makes it necessary for their safety. Patients with liver metastases
amenable to hepatic resection or resection and ablation to render them “disease
free” are ineligible for this study.
Some other, subtle criteria relate to the treatment, similar to the criteria for
the NSABP-C-08 trial that evaluated adjuvant fluorouracil, leucovorin and
oxaliplatin with or without bevacizumab. For example, we don’t want patients
whose risk would be increased with the use of bevacizumab, such as patients
with active ulcer disease or arterial events or MIs within the past six months.
 DR LOVE: What are the primary endpoints for the study?
DR LOVE: What are the primary endpoints for the study?
 DR WOLMARK: The endpoints consist of monitoring the rate of operations for
complications of the primary tumor, such as bleeding, perforation, fistula or
obstruction, or death related to the primary tumor.
DR WOLMARK: The endpoints consist of monitoring the rate of operations for
complications of the primary tumor, such as bleeding, perforation, fistula or
obstruction, or death related to the primary tumor.
The trial is powered specifically to determine outcomes relative to the
primary tumor. If the incidence of events is less than 25 percent, the therapeutic
intervention will be considered a success, but if the event rate is higher,
it will be considered unsuccessful.
We plan to accrue 90 patients, and to safeguard patients, we are using a
Simon two-stage design. Initially we will enroll 30 patients, in order to have
26 evaluable patients, and then the trial goes into hiatus so that we can determine
the event rate. If 10 or more patients have experienced an event — death
from the primary or surgery or bleeding, perforation, fistula or obstruction
related to the primary — the trial will be stopped. If fewer than 10 patients
have experienced an event, the study will continue and we will enter the next
60 patients.
In addition, these are patients who are deemed inoperable, so a tertiary
endpoint is to determine how many patients can be converted from inoperable to operable, in terms of metastatic disease and the primary, with a modern
regimen such as FOLFOX6 with bevacizumab.
Track 4
 DR LOVE:
DR LOVE: Axel, how do you treat patients with metastatic colorectal
cancer and an intact primary tumor?
 DR GROTHEY: The BRiTE registry followed prospectively approximately
2,000 patients treated with chemotherapy and bevacizumab for metastatic
colorectal cancer, and about 16 percent of those patients had the primary
intact.
DR GROTHEY: The BRiTE registry followed prospectively approximately
2,000 patients treated with chemotherapy and bevacizumab for metastatic
colorectal cancer, and about 16 percent of those patients had the primary
intact.
They revealed that 3.4 percent of these patients experienced gastrointestinal
perforations, which is about twice as high as in the overall registry population,
but of course they eliminated the risks of primary surgery, so it’s a tradeoff.
Currently, I believe that if the metastases are the dominating life-threatening
factor, I start with chemotherapy right away, with the primary intact. Also, I
do use bevacizumab in that situation.
 DR LOVE: Dan, if it were breast cancer, we would leave the primary intact and
use it as an indicator lesion for systemic therapy of metastases. Does that strategy
make sense in colorectal cancer?
DR LOVE: Dan, if it were breast cancer, we would leave the primary intact and
use it as an indicator lesion for systemic therapy of metastases. Does that strategy
make sense in colorectal cancer?
 DR HALLER: I believe it does. However, this approach is highly individualized,
so it’s between the patient and the surgeon to make the final decision. If
a patient has a nonobstructing, nonbleeding primary tumor, more often than
not, we leave in place.
DR HALLER: I believe it does. However, this approach is highly individualized,
so it’s between the patient and the surgeon to make the final decision. If
a patient has a nonobstructing, nonbleeding primary tumor, more often than
not, we leave in place.
 DR LOVE: Rich, how do you manage these cases?
DR LOVE: Rich, how do you manage these cases?
 DR GOLDBERG: My default position is not to operate on patients who present
with widely metastatic disease unless they’re obstructed or bleeding, and I
have been willing to administer bevacizumab.
DR GOLDBERG: My default position is not to operate on patients who present
with widely metastatic disease unless they’re obstructed or bleeding, and I
have been willing to administer bevacizumab.
 DR LOVE: Do you see any difference in response to therapy in the primary
tumor compared to what you typically see with metastatic disease?
DR LOVE: Do you see any difference in response to therapy in the primary
tumor compared to what you typically see with metastatic disease?
 DR GOLDBERG: The primaries seem to be quite responsive, and it’s been
interesting to follow them.
DR GOLDBERG: The primaries seem to be quite responsive, and it’s been
interesting to follow them.
 DR WOLMARK: That’s precisely why I believe the NSABP-C-10 trial will
provide us with useful information.
DR WOLMARK: That’s precisely why I believe the NSABP-C-10 trial will
provide us with useful information.
 DR GROTHEY: This point that the primary tumor can respond to therapy is
important because some surgeons don’t believe that. Norm, you’re an exception,
but I routinely hear at the Mayo Clinic that primaries don’t respond and
we need to perform surgery. That is not true.
DR GROTHEY: This point that the primary tumor can respond to therapy is
important because some surgeons don’t believe that. Norm, you’re an exception,
but I routinely hear at the Mayo Clinic that primaries don’t respond and
we need to perform surgery. That is not true.
 DR HOCHSTER: That’s been our experience also. In fact, a couple of patients
who’ve undergone post-therapy colonoscopy have had unobservable primaries,
so I believe the primary is often more sensitive than the liver metastases.
In terms of bleeding, I have yet to see any patient with metastatic colorectal
cancer and an intact primary tumor experience a major bleeding problem.
DR HOCHSTER: That’s been our experience also. In fact, a couple of patients
who’ve undergone post-therapy colonoscopy have had unobservable primaries,
so I believe the primary is often more sensitive than the liver metastases.
In terms of bleeding, I have yet to see any patient with metastatic colorectal
cancer and an intact primary tumor experience a major bleeding problem.
 DR MEROPOL: I agree with the other panel members in that I tend to defer
surgery in general, and I’m comfortable using bevacizumab as long as there
isn’t a lot of bleeding.
DR MEROPOL: I agree with the other panel members in that I tend to defer
surgery in general, and I’m comfortable using bevacizumab as long as there
isn’t a lot of bleeding.
Select Publications

