
| Tracks 1-9 |
| Track 1 |
Use of oxaliplatin as a radio-sensitizer, systemic therapy or both |
| Track 2 |
NSABP-R-04: Preoperative radiation therapy and capecitabine or 5-FU with or without oxaliplatin for operable rectal cancer |
| Track 3 |
Use of preoperative versus postoperative chemotherapy in rectal cancer |
| Track 4 |
Importance of preoperative
staging in the determination
of optimal therapy for
rectal cancer |
|
| Track 5 |
Preoperative chemoradiation therapy with oxaliplatin for select patients with locally advanced rectal cancer |
| Track 6 |
Rationale for NSABP-R-04 design |
| Track 7 |
Preoperative staging and the
use of neoadjuvant
chemoradiation therapy versus
postoperative chemotherapy |
| Track 8 |
Incorporating biologic agents in preoperative therapy of rectal cancer |
| Track 9 |
Use of preoperative oxaliplatin-containing
chemoradiation
therapy for rectal cancer |
|
|
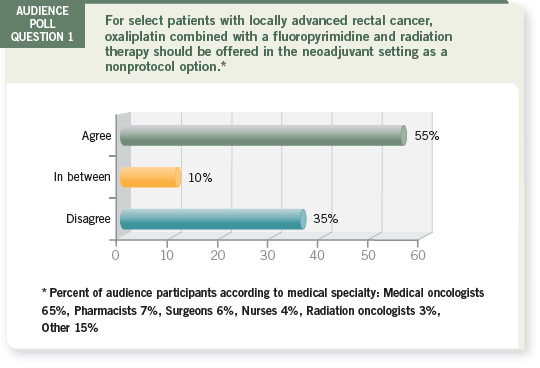
Track 1
 DR LOVE:
DR LOVE: Dan, how would you respond to this poll question about
oxaliplatin as neoadjuvant therapy — agree, disagree or in between?
 DR HALLER: I would say “in between” because I wear two different hats:
one as a person who conducted a Phase I/II trial in this area and uses it off
protocol and another as the GI Intergroup co-chair with a protocol (NSABPR-04; [1.2]) specifically addressing this question.
DR HALLER: I would say “in between” because I wear two different hats:
one as a person who conducted a Phase I/II trial in this area and uses it off
protocol and another as the GI Intergroup co-chair with a protocol (NSABPR-04; [1.2]) specifically addressing this question.
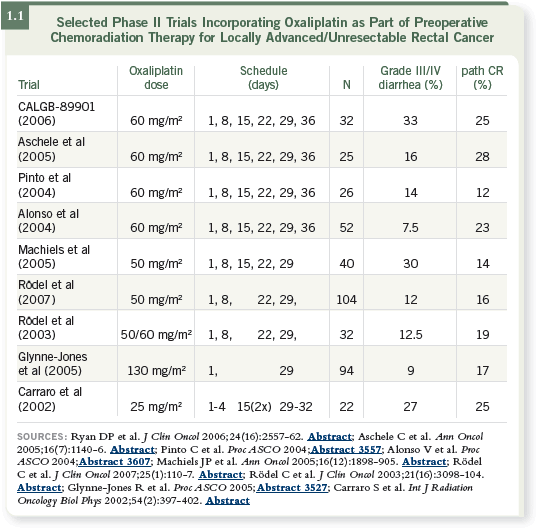
Separate Phase I or II studies evaluating this regimen have been conducted
worldwide (1.1), and some clear conclusions have emerged. First, it is evident
that you can add oxaliplatin to a fluoropyrimidine and radiation therapy on
a weekly or biweekly schedule or once every three weeks and at different
doses. The dose depends on whether you consider oxaliplatin a radiosensitizer,
a systemic agent or both. That is, you’re administering adjuvant therapy and
local therapy to make the radiation therapy work better.
If you want to use oxaliplatin as a radiosensitizer, you probably want to use it
more frequently, as in CALGB-89901 (Ryan 2006). If you believe it’s more of
a systemic agent, you certainly want to administer the maximal dose.
It turns out if you administer oxaliplatin weekly, not everybody gets through
all the dosing. So although the total dose that could be delivered is high, the
actual dose administered with the weekly regimens is much lower than with
the every two-week or every three-week regimens. We have defaulted to a
weekly regimen because CALGB data suggested that oxaliplatin was more of a
radiosensitizer.
In the German preoperative trial, the pathologic complete response (CR) rate
was found to correlate with disease-free survival (Rödel 2005). For patients
who have received radiation therapy alone or radiation therapy with 5-FU, the
pathologic CR rate is in the range of about eight to 10 percent (Sauer 2004).
In the 5-FU/oxaliplatin studies, the pathologic CR rates range anywhere from
about 18 percent to 48 percent.
Track 2
 DR LOVE:
DR LOVE: Can you discuss the NSABP-R-04 trial design?
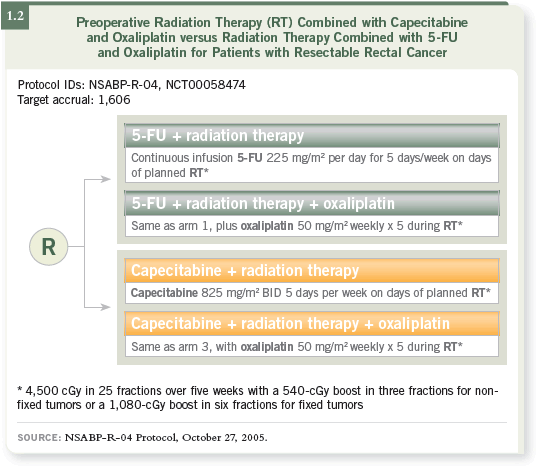
 DR HALLER: The primary comparison in NSABP-R-04 was capecitabine
versus infusional 5-FU (1.2). It started to become a fairly pedestrian question
because many physicians had already diverted to using capecitabine. However,
it remains an important question because you have to lower the dose of
capecitabine from the “standard systemic dose.” You need to ensure that you’re
maintaining — for a patient being treated with curative intent — the same
rates obtained with infusional 5-FU.
DR HALLER: The primary comparison in NSABP-R-04 was capecitabine
versus infusional 5-FU (1.2). It started to become a fairly pedestrian question
because many physicians had already diverted to using capecitabine. However,
it remains an important question because you have to lower the dose of
capecitabine from the “standard systemic dose.” You need to ensure that you’re
maintaining — for a patient being treated with curative intent — the same
rates obtained with infusional 5-FU.
 DR LOVE: What is the dosing schedule for capecitabine in NSABP-R-04?
DR LOVE: What is the dosing schedule for capecitabine in NSABP-R-04?
 DR HALLER: It’s 825 mg/m2 twice a day on Monday through Friday. As the
study was just about to be launched, the whole oxaliplatin issue came along.
Many of us thought the trial would be better as a two-by-two design asking
two important questions. We will have the definitive answer from NSABPR-
04, but I would guess that the oxaliplatin combinations would have better
pathologic CR rates. Whether that translates into better long-term outcomes
— including acute and late toxicities, sphincter preservation, type of surgery
and overall survival — is where NSABP-R-04 comes into play.
DR HALLER: It’s 825 mg/m2 twice a day on Monday through Friday. As the
study was just about to be launched, the whole oxaliplatin issue came along.
Many of us thought the trial would be better as a two-by-two design asking
two important questions. We will have the definitive answer from NSABPR-
04, but I would guess that the oxaliplatin combinations would have better
pathologic CR rates. Whether that translates into better long-term outcomes
— including acute and late toxicities, sphincter preservation, type of surgery
and overall survival — is where NSABP-R-04 comes into play.
 DR LOVE: What do we know about the safety and tolerability of adding oxaliplatin
to a fluoropyrimidine in the neoadjuvant setting?
DR LOVE: What do we know about the safety and tolerability of adding oxaliplatin
to a fluoropyrimidine in the neoadjuvant setting?
 DR HALLER: In most of the studies, more diarrhea occurred with the weekly
regimen, which is why more doses were dropped (1.1). Patients received more oxaliplatin when it was administered less frequently because they didn’t face
dose reductions and delays.
In treating patients with rectal cancer, it’s important that you not interfere
with the radiation therapist administering treatment at the standard dose and
schedule. If you had six treatment interruptions for hospitalizations and such,
you may see an inferior outcome because the radiation therapy was unintentionally
administered in a split-course fashion.
DR HALLER: In most of the studies, more diarrhea occurred with the weekly
regimen, which is why more doses were dropped (1.1). Patients received more oxaliplatin when it was administered less frequently because they didn’t face
dose reductions and delays.
In treating patients with rectal cancer, it’s important that you not interfere
with the radiation therapist administering treatment at the standard dose and
schedule. If you had six treatment interruptions for hospitalizations and such,
you may see an inferior outcome because the radiation therapy was unintentionally
administered in a split-course fashion.
Track 5
 DR LOVE:
DR LOVE: Axel, what are your thoughts about the audience’s response to
the poll question?
 DR GROTHEY: I was intrigued that approximately two thirds of the people
here tonight would consider oxaliplatin — at least for some patients — outside
of a clinical trial, in the neoadjuvant setting with, so far, only Phase II data.
The question I pose to Dan is, do we really need the data from the NSABPR-04 study if two thirds of us are already using this approach?
DR GROTHEY: I was intrigued that approximately two thirds of the people
here tonight would consider oxaliplatin — at least for some patients — outside
of a clinical trial, in the neoadjuvant setting with, so far, only Phase II data.
The question I pose to Dan is, do we really need the data from the NSABPR-04 study if two thirds of us are already using this approach?
 DR HALLER: We certainly know that capecitabine is a feasible agent, but the
dose that you can administer during radiation therapy is considerably lower
than the dose you would normally administer systemically to most patients.
So I do want to see that the lower capecitabine dose is equivalent to infusional
5-FU.
DR HALLER: We certainly know that capecitabine is a feasible agent, but the
dose that you can administer during radiation therapy is considerably lower
than the dose you would normally administer systemically to most patients.
So I do want to see that the lower capecitabine dose is equivalent to infusional
5-FU.
Track 9
 DR LOVE:
DR LOVE: Rich, are there situations in which you would use oxaliplatin
in this setting?
 DR GOLDBERG: We participated in the CALGB study, and I had several
patients who showed good responses to oxaliplatin and 5-FU on protocol. I
participate in the NSABP-R-04 trial, but I don’t use oxaliplatin in this setting
off study.
DR GOLDBERG: We participated in the CALGB study, and I had several
patients who showed good responses to oxaliplatin and 5-FU on protocol. I
participate in the NSABP-R-04 trial, but I don’t use oxaliplatin in this setting
off study.
 DR GROTHEY: I’ve used oxaliplatin outside of clinical trials in the neoadjuvant
setting for patients with large tumors for whom I wanted to use the radiosensitization
activity of oxaliplatin. Also, for patients who present with presumably
curable metastatic disease, this utilizes the most active therapy up front and
still provides patients with the benefits of local tumor control, preservation of
sphincter function, et cetera.
DR GROTHEY: I’ve used oxaliplatin outside of clinical trials in the neoadjuvant
setting for patients with large tumors for whom I wanted to use the radiosensitization
activity of oxaliplatin. Also, for patients who present with presumably
curable metastatic disease, this utilizes the most active therapy up front and
still provides patients with the benefits of local tumor control, preservation of
sphincter function, et cetera.
 DR HOCHSTER: For a couple of patients who have presented in my practice
like that, we’ve used FOLFOX without bevacizumab and saved radiation
therapy for postoperative treatment. The question is about the combination
with radiation. What will the increased acute and the long-term toxicities be
when you add these agents and a biologic together with radiation therapy?
DR HOCHSTER: For a couple of patients who have presented in my practice
like that, we’ve used FOLFOX without bevacizumab and saved radiation
therapy for postoperative treatment. The question is about the combination
with radiation. What will the increased acute and the long-term toxicities be
when you add these agents and a biologic together with radiation therapy?
Select Publications

