
 |
|||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
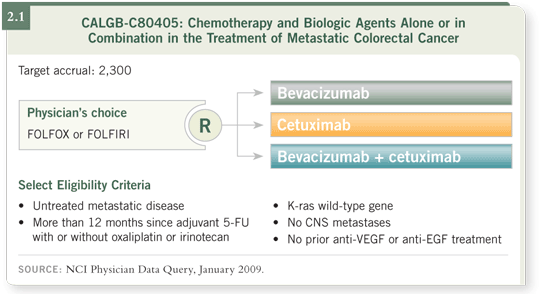
![]() DR LOVE: Let’s discuss this case. In CALGB-C80405, the clinician can select either FOLFOX or FOLFIRI. Why did you choose FOLFIRI?
DR LOVE: Let’s discuss this case. In CALGB-C80405, the clinician can select either FOLFOX or FOLFIRI. Why did you choose FOLFIRI?
![]() DR LENZ: The choice between FOLFOX and FOLFIRI as first-line therapy for metastatic colorectal cancer is controversial. I believe that in the community these combinations are interchangeable in terms of efficacy and toxicity.
DR LENZ: The choice between FOLFOX and FOLFIRI as first-line therapy for metastatic colorectal cancer is controversial. I believe that in the community these combinations are interchangeable in terms of efficacy and toxicity.
During the past few years, we have established a molecular signature that predicts response. With certain genes, FOLFOX does not work and FOLFIRI has a higher chance of providing benefit. We tested the patient for two genes that predict for sensitivity to 5-FU and oxaliplatin — thymidylate synthase (TS) and excision repair cross-complementation group 1 (ERCC1). The latter has been tested prospectively and validated in non-small cell lung cancer (Bepler 2008).
Patients with colorectal cancer and high levels of ERCC1 are unlikely to benefit from platinum-based chemotherapy (Uchida 2008) and may benefit more from irinotecan (Vallböhmer 2006). In this patient, the TS and ERCC1 were so high that I knew oxaliplatin was unlikely to be of any benefit. In the United States, most oncologists would have chosen FOLFOX/bevacizumab, which is the most used combination regimen. We chose FOLFIRI, however, because this patient had high levels of TS and ERCC1.
Tracks 8-9
![]() DR LOVE: This patient is receiving double antibody therapy, which has become controversial. As one of the investigators in the BOND-2 trial, what’s your view of this strategy?
DR LOVE: This patient is receiving double antibody therapy, which has become controversial. As one of the investigators in the BOND-2 trial, what’s your view of this strategy?
![]() DR LENZ: In BOND-2, patients with refractory colorectal cancer were randomly assigned to irinotecan/cetuximab/bevacizumab versus cetuximab/bevacizumab. In these patients with refractory disease, both monoclonal antibodies demonstrated significant efficacy, which was enhanced by the addition of irinotecan (Saltz 2007).
DR LENZ: In BOND-2, patients with refractory colorectal cancer were randomly assigned to irinotecan/cetuximab/bevacizumab versus cetuximab/bevacizumab. In these patients with refractory disease, both monoclonal antibodies demonstrated significant efficacy, which was enhanced by the addition of irinotecan (Saltz 2007).
This clinical trial is striking not only because of significant efficacy in patients with highly refractory disease but also because of low toxicity. No overlapping or additional toxicities were encountered (Saltz 2007). I was surprised when the initial data were reported from the first-line trials, PACCE and CAIRO-2.
In the PACCE trial, the investigator had a choice between FOLFOX and FOLFIRI. Patients were then randomly assigned to bevacizumab versus bevacizumab/panitumumab (Hecht 2008a, 2008b). CAIRO-2 evaluated CAPOX/bevacizumab with or without cetuximab (Punt 2008).
Among the patients receiving both monoclonal antibodies, the trials demonstrated similar patterns of increased toxicity and a decrease in efficacy, as measured by response rate and progression-free survival (Hecht 2008a; Punt 2008). One explanation for these results is that K-ras sorts out patients who benefit from cetuximab or panitumumab, and the particular chemotherapeutic agent may also play a significant role. But K-ras alone will not explain the differences in toxicity and efficacy.
As we have seen in other clinical trials, particularly those with oxaliplatin-based combinations with bevacizumab, some patients may be harmed by increasing resistance to certain chemotherapies when cetuximab is combined with bevacizumab.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2009 Research To Practice. All Rights Reserved. |