
 |
|||||||

| Tracks 1-10 | ||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Would you discuss the CONcePT trial?
DR LOVE: Would you discuss the CONcePT trial?
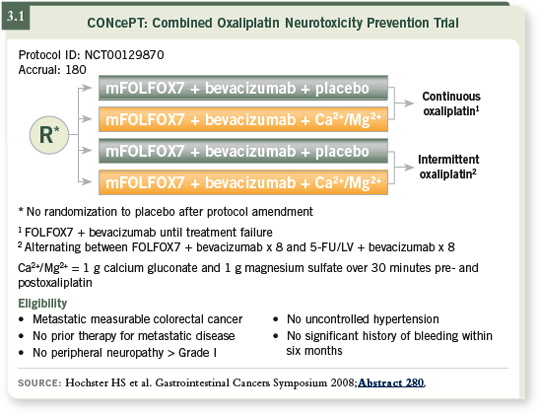
![]() DR HOCHSTER: Our goal was to conduct the first randomized, placebo-controlled study of calcium/magnesium for the prevention of oxaliplatin-induced neurotoxicity. A second goal was to improve the time to treatment failure (Hochster 2008; [3.1]).
DR HOCHSTER: Our goal was to conduct the first randomized, placebo-controlled study of calcium/magnesium for the prevention of oxaliplatin-induced neurotoxicity. A second goal was to improve the time to treatment failure (Hochster 2008; [3.1]).
We knew about the scheduling issue with oxaliplatin from Dr de Gramont’s OPTIMOX1 study, which used a high dose of oxaliplatin for six cycles and then discontinued it until the patient’s disease progressed (Tournigand 2006). In the CONcePT study, we used a conventional dose of oxaliplatin but administered it in four-month blocks (intermittent oxaliplatin). Patients received four months of FOLFOX with bevacizumab, four months of 5-FU with bevacizumab and then automatically restarted oxaliplatin at the eighth month (Hochster 2008; [3.1]).
Accrual to the trial was slow, and most doctors did not believe we needed a randomized study of calcium/magnesium because they all knew it worked. So we amended the protocol after 140 patients had enrolled and allowed everyone to receive calcium/magnesium. After that amendment, the trial involved only the randomization to either intermittent or continuous oxaliplatin (Hochster 2008).
After 180 patients enrolled, the Independent Data Monitoring Committee (IDMC) reviewed the data. It appeared that fewer responses occurred in the group who had been receiving calcium/magnesium. At that point, the IDMC stopped the trial and didn’t allow anyone to receive calcium/magnesium.
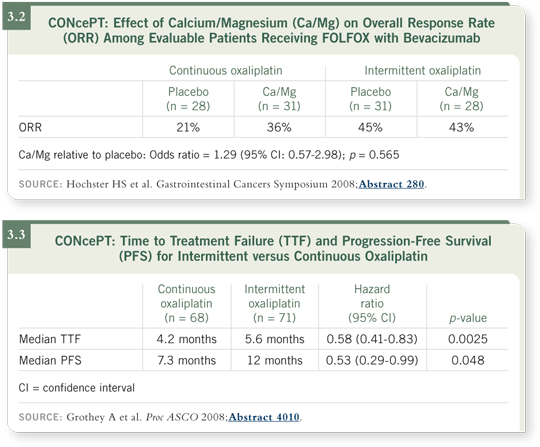
In the meantime, we submitted all of the scans to an independent radiology review committee. As we presented at the 2008 Gastrointestinal Cancers Symposium, the response rate was equivalent or slightly better for the patients receiving calcium/magnesium, with an odds ratio of 1.3 (Hochster 2008; [3.2]).
Furthermore, we reported that the hazard ratio for time to treatment failure was approximately 0.6 in favor of intermittent oxaliplatin. Instead of about four months with continuous oxaliplatin, the median time to treatment failure — the primary endpoint — was about six months for the group who received intermittent oxaliplatin.
The difference in median progression-free survival was even longer — seven versus 12 months. With a truncated study of only 140 patients, the difference was large enough to indicate a real effect on time to treatment failure and progression-free survival (Grothey 2008; [3.3]).
Tracks 4-5
![]() DR LOVE: What’s your conclusion?
DR LOVE: What’s your conclusion?
![]() DR HOCHSTER: I believe the data are clear that calcium/magnesium does not interfere with the activity of FOLFOX and that its use does reduce cumulative and acute neurotoxicity. So I believe it’s safe to use. Those who found calcium/magnesium helpful before should not be concerned about readopting it as a standard approach.
DR HOCHSTER: I believe the data are clear that calcium/magnesium does not interfere with the activity of FOLFOX and that its use does reduce cumulative and acute neurotoxicity. So I believe it’s safe to use. Those who found calcium/magnesium helpful before should not be concerned about readopting it as a standard approach.
![]() DR LOVE: Do you use calcium/magnesium preventively in your practice?
DR LOVE: Do you use calcium/magnesium preventively in your practice?
![]() DR HOCHSTER: I do. I have gone back to using it all the time. I had stopped for about eight to 10 months while we were assessing the data from the CONcePT study.
DR HOCHSTER: I do. I have gone back to using it all the time. I had stopped for about eight to 10 months while we were assessing the data from the CONcePT study.
![]() DR LOVE: Do you use it routinely for every patient in the adjuvant and metastatic settings?
DR LOVE: Do you use it routinely for every patient in the adjuvant and metastatic settings?
![]() DR HOCHSTER: In the metastatic setting, we have the response data. If somebody wants to be a stickler, they might say that we don’t know that an effect is evident in the adjuvant setting. So it could be more risky to use it in the adjuvant setting, but I do not see how that’s a possibility.
DR HOCHSTER: In the metastatic setting, we have the response data. If somebody wants to be a stickler, they might say that we don’t know that an effect is evident in the adjuvant setting. So it could be more risky to use it in the adjuvant setting, but I do not see how that’s a possibility.
![]() DR LOVE: What about the use of intermittent oxaliplatin as evaluated in CONcePT?
DR LOVE: What about the use of intermittent oxaliplatin as evaluated in CONcePT?
![]() DR HOCHSTER: I believe to obtain the maximum benefit from oxaliplatin it is important to take a break and then use it again. In CONcePT, we used four months on, four months off and four months on again. Patients demonstrated a 12-month median time to disease progression, which is what we thought would happen when we added bevacizumab (Grothey 2008; [3.3]). This is a way to derive more benefit from oxaliplatin.
DR HOCHSTER: I believe to obtain the maximum benefit from oxaliplatin it is important to take a break and then use it again. In CONcePT, we used four months on, four months off and four months on again. Patients demonstrated a 12-month median time to disease progression, which is what we thought would happen when we added bevacizumab (Grothey 2008; [3.3]). This is a way to derive more benefit from oxaliplatin.
Track 10
![]() DR LOVE: Would you discuss the selection of therapy for a patient for whom you would like to downstage unresectable liver metastases?
DR LOVE: Would you discuss the selection of therapy for a patient for whom you would like to downstage unresectable liver metastases?
![]() DR HOCHSTER: We don’t have particularly helpful data indicating whether FOLFOX or FOLFIRI is the preferable regimen in this setting — I believe either can be effective. The real issue today might be the use of bevacizumab or cetuximab. Tumor shrinkage with bevacizumab may not be as much as we’d like.
DR HOCHSTER: We don’t have particularly helpful data indicating whether FOLFOX or FOLFIRI is the preferable regimen in this setting — I believe either can be effective. The real issue today might be the use of bevacizumab or cetuximab. Tumor shrinkage with bevacizumab may not be as much as we’d like.
In the large NO16966 study, the response rate was the same for FOLFOX with or without bevacizumab (Saltz 2008). However, in the CRYSTAL trial — a first-line study of FOLFIRI with or without cetuximab — patients with wild-type K-ras benefited and their response rate was augmented with the addition of cetuximab (Van Cutsem 2008).
So the real question is, should we be using cetuximab as our first-line antibody if we’re trying to shrink K-ras wild-type liver metastases preoperatively?
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2009 Research To Practice. All Rights Reserved. |