
 |
|||||||

| Tracks 1-10 | ||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 4-5
![]() DR LOVE: What are your thoughts about the choice of systemic therapy to downstage K-ras wild-type unresectable liver-only metastases?
DR LOVE: What are your thoughts about the choice of systemic therapy to downstage K-ras wild-type unresectable liver-only metastases?
![]() DR HURWITZ: Based on the BRiTE registry in the United States and the First BEAT trial in Europe, approximately 10 percent of patients treated with FOLFOX/bevacizumab undergo surgery with curative intent and 80 percent or more of those have an R0 resection (ie, no disease left; [Cassidy 2008]). With FOLFIRI/cetuximab, the downstaging rate is in the five to 10 percent range and the R0 rate is 60 to 70 percent.
DR HURWITZ: Based on the BRiTE registry in the United States and the First BEAT trial in Europe, approximately 10 percent of patients treated with FOLFOX/bevacizumab undergo surgery with curative intent and 80 percent or more of those have an R0 resection (ie, no disease left; [Cassidy 2008]). With FOLFIRI/cetuximab, the downstaging rate is in the five to 10 percent range and the R0 rate is 60 to 70 percent.
All this means is that active first-line chemotherapy will downstage disease in some patients. I do not believe the available data allow us to make any inferences related to which chemotherapy regimen is better. Clinicians should simply choose what they consider to be their most active or preferred chemotherapy regimen.
![]() DR LOVE: What is your preferred regimen in this situation?
DR LOVE: What is your preferred regimen in this situation?
![]() DR HURWITZ: Barring contraindications, I prefer FOLFOX or CAPOX with bevacizumab. Bevacizumab is currently supported by more data suggesting benefit, including a survival benefit. For a patient with a good performance status who is not a candidate for a VEGF inhibitor, such as one with a history of cardiac symptoms, cetuximab in the first-line setting may make sense.
DR HURWITZ: Barring contraindications, I prefer FOLFOX or CAPOX with bevacizumab. Bevacizumab is currently supported by more data suggesting benefit, including a survival benefit. For a patient with a good performance status who is not a candidate for a VEGF inhibitor, such as one with a history of cardiac symptoms, cetuximab in the first-line setting may make sense.
Although wound complications are an issue with bevacizumab, the risk is small. Most hepatic surgeons want patients to be off of chemotherapy for at least one month before surgery to let the liver recover from any treatment-related side effects and to improve the patient’s performance status. The time off of bevacizumab should probably be consistent with its half-life, and the goal is approximately six to eight weeks. With that time frame, a significant increase in surgical risk is not evident.
Postoperatively, I don’t believe any undue risk ensues in resuming chemotherapy or bevacizumab once the patient is well healed, which is usually eight weeks after a liver resection. The initial safety report from NSABP-C-08, evaluating adjuvant FOLFOX with bevacizumab, demonstrated a one to two percent increase in wound complications associated with bevacizumab (Allegra 2008; [1.2]). That rate was largely a result of hernia formation, which in the context of a potentially curative resection should be manageable.
Track 8
![]() DR LOVE: Would you comment on the BRiTE registry data evaluating the use of bevacizumab beyond first disease progression?
DR LOVE: Would you comment on the BRiTE registry data evaluating the use of bevacizumab beyond first disease progression?
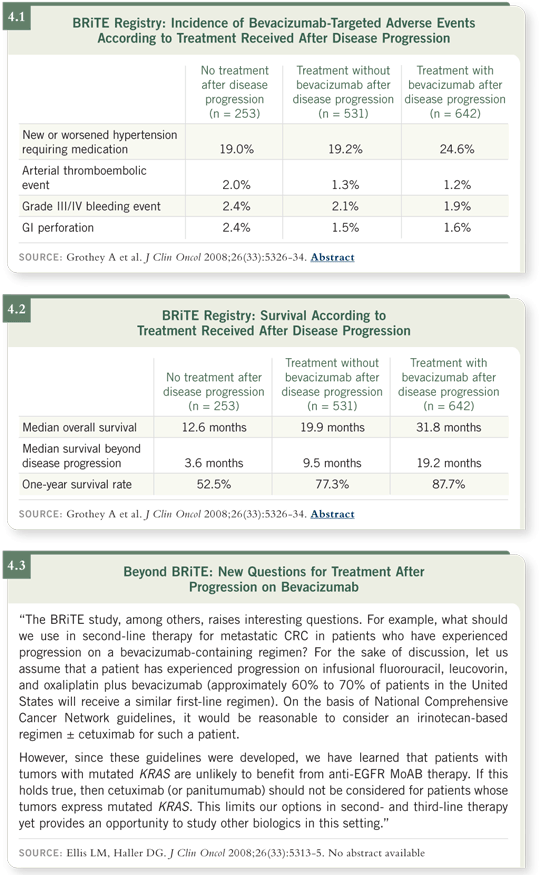
![]() DR HURWITZ: The BRiTE registry was established to follow patients who received first-line therapy with bevacizumab in order to define rare toxicities, such as cardiovascular issues and wound healing (Grothey 2008; [4.1]). We can’t estimate the actual rate of these problems or identify contributing factors without evaluating thousands of patients.
DR HURWITZ: The BRiTE registry was established to follow patients who received first-line therapy with bevacizumab in order to define rare toxicities, such as cardiovascular issues and wound healing (Grothey 2008; [4.1]). We can’t estimate the actual rate of these problems or identify contributing factors without evaluating thousands of patients.
The registry also gathered some outcomes data and found that patients who continued to receive bevacizumab past first disease progression fared better than those who did not (Grothey 2008; [4.2]; Ellis 2008; [4.3]). I believe this finding is consistent with two phenomena. One is that patients with more indolent disease experience progression more slowly and fare better if you continue their treatment. The other is that additional bevacizumab after initial disease progression is beneficial. It might be that bevacizumab is working best in the patients who have more indolent disease.
The BRiTE registry data also emphasize that what we call disease progression in a clinical trial may be defined differently in clinical practice. In practice, if a patient’s disease has a relatively slow progression during the course of two years, although the measurements may technically meet RECIST criteria for progression, the clinician may judge the rate of progression to be slow enough to say that the patient is benefiting from therapy and that clinician may choose to continue one part of the regimen while adjusting other elements.
Track 9
![]() DR LOVE: How do you approach the continuation of bevacizumab and the use of treatment holidays in metastatic disease?
DR LOVE: How do you approach the continuation of bevacizumab and the use of treatment holidays in metastatic disease?
![]() DR HURWITZ: I expect the best answer to how long to administer bevacizumab will come from prospective studies that randomly assign patients to continuation or noncontinuation. The separate question of how to navigate treatment holidays is an excellent one now that patients are likely to receive first-line therapy for nine to 12 months and, if they’re faring particularly well, even longer.
DR HURWITZ: I expect the best answer to how long to administer bevacizumab will come from prospective studies that randomly assign patients to continuation or noncontinuation. The separate question of how to navigate treatment holidays is an excellent one now that patients are likely to receive first-line therapy for nine to 12 months and, if they’re faring particularly well, even longer.
We often need to manage the side effects of some or all of the drugs. Both oxaliplatin and irinotecan can be difficult drugs to continue past four to six months. Oxaliplatin causes neuropathy and cumulative asthenia, and patients can experience asthenia and diarrhea with irinotecan.
Therefore, we almost certainly have to navigate a holiday or what I call a “working holiday,” which involves the continuation of the parts of the regimen that are still tolerable. So FOLFOX/bevacizumab becomes 5-FU/bevacizumab and FOLFIRI/bevacizumab becomes 5-FU/bevacizumab.
I believe the best data for the use of bevacizumab suggest that it works better with some chemotherapy, so I have a bias toward maintaining 5-FU. If they can’t do that, I believe clinicians should use their best judgment about how to make the next gradation of a holiday. Few data exist for bevacizumab as monotherapy, and I tend to offer my patients a complete break. Whether you continue with no treatment, bevacizumab or 5-FU/bevacizumab, you’re still observing the patient. If his or her CEA rises or a tumor size increases on CAT scans, then you can adjust and add more therapy or move on to your next treatment.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2009 Research To Practice. All Rights Reserved. |