
 |
|||||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the tyrosine kinase inhibitors (TKIs) that are being investigated in the treatment of colorectal cancer?
DR LOVE: Would you discuss the tyrosine kinase inhibitors (TKIs) that are being investigated in the treatment of colorectal cancer?
![]() DR HOFF: Two TKIs are currently being evaluated in Phase III trials in colorectal cancer. A large international trial is evaluating FOLFIRI with or without sunitinib — which blocks VEGF and PDGF receptors — as first-line therapy for metastatic disease (A6181122). Because the trial includes one arm without a biologic agent, it’s primarily being conducted outside of the United States, where that approach is more acceptable.
DR HOFF: Two TKIs are currently being evaluated in Phase III trials in colorectal cancer. A large international trial is evaluating FOLFIRI with or without sunitinib — which blocks VEGF and PDGF receptors — as first-line therapy for metastatic disease (A6181122). Because the trial includes one arm without a biologic agent, it’s primarily being conducted outside of the United States, where that approach is more acceptable.
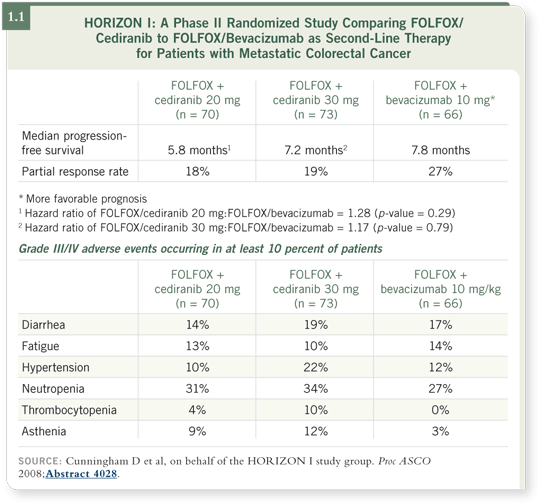
The other TKI under investigation, cediranib, blocks VEGFR-1, VEGFR-2, VEGFR-3 and c-Kit. Data from HORIZON I, a Phase II randomized trial evaluating second-line therapy with FOLFOX/bevacizumab versus FOLFOX/cediranib at 20 milligrams per day versus FOLFOX/cediranib at 30 milligrams per day, were reported at ASCO 2008 (Cunningham 2008).
Although no statistical difference in progression-free survival was evident among the three arms, the data suggest that cediranib has clinical activity in the same range as bevacizumab. The response rate was numerically better for FOLFOX/bevacizumab but was not statistically different (Cunningham 2008; [1.1]).
Two Phase III trials are currently evaluating cediranib in the first-line setting. One, being conducted outside of the United States, originally evaluated two different doses of cediranib. After the HORIZON I data became available, the trial was modified to evaluate FOLFOX with placebo or cediranib at 20 milligrams per day. This trial has recently completed accrual of 1,050 patients.
The other trial, HORIZON III, is being conducted in the United States and compares FOLFOX/bevacizumab to FOLFOX/cediranib at 20 milligrams per day. This trial has a target accrual of 1,600 patients, which should be completed within the next few months. We may have efficacy data from both of these trials by late 2009 or early 2010.
Track 2
![]() DR LOVE: How do the side effects of cediranib compare to those of bevacizumab?
DR LOVE: How do the side effects of cediranib compare to those of bevacizumab?
![]() DR HOFF: The toxicity profiles from two arms of the HORIZON I trial, FOLFOX/bevacizumab and FOLFOX/cediranib at 20 milligrams per day, are similar. However, some differences are evident.
DR HOFF: The toxicity profiles from two arms of the HORIZON I trial, FOLFOX/bevacizumab and FOLFOX/cediranib at 20 milligrams per day, are similar. However, some differences are evident.
Numerically at least, patients taking cediranib experience somewhat more asthenia (Cunningham 2008; [1.1]). In my practice, patients mention more dysphonia and hoarseness with cediranib, although this has not been a major problem. The other side effects we see with cediranib are those you would expect from an anti-VEGF agent, such as hypertension.
We believe this is a class effect. How the hypertension associated with bevacizumab compares to that induced by cediranib will be better answered by the larger HORIZON III trial with 1,600 patients.
Tracks 3-5, 7
![]() DR LOVE: Would you discuss the design of the AVANT trial and when you expect the data will be available?
DR LOVE: Would you discuss the design of the AVANT trial and when you expect the data will be available?
![]() DR HOFF: The AVANT study was designed to explore the use of adjuvant bevacizumab in patients with Stage III or high-risk Stage II colon cancer. Patients were randomly assigned to receive FOLFOX, FOLFOX/bevacizumab or CAPOX/bevacizumab. We accrued 3,450 patients, and we don’t expect results until perhaps 2010 or early 2011.
DR HOFF: The AVANT study was designed to explore the use of adjuvant bevacizumab in patients with Stage III or high-risk Stage II colon cancer. Patients were randomly assigned to receive FOLFOX, FOLFOX/bevacizumab or CAPOX/bevacizumab. We accrued 3,450 patients, and we don’t expect results until perhaps 2010 or early 2011.
![]() DR LOVE: What were your thoughts about the toxicity data presented at ASCO 2008 on the NSABP-C-08 trial?
DR LOVE: What were your thoughts about the toxicity data presented at ASCO 2008 on the NSABP-C-08 trial?
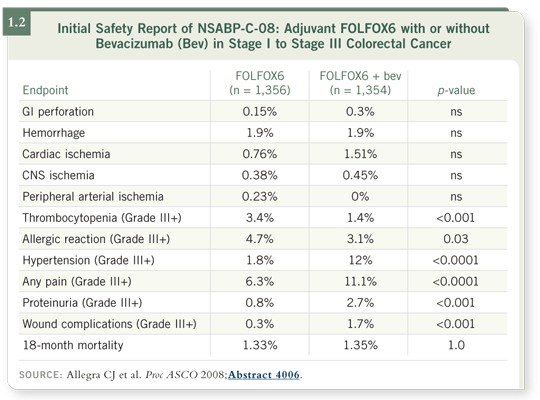
![]() DR HOFF: No substantial increase in toxicity was observed with the addition of bevacizumab to adjuvant FOLFOX. I found it particularly interesting that the rate of thrombocytopenia decreased among the patients who received bevacizumab (Allegra 2008; [1.2]).
DR HOFF: No substantial increase in toxicity was observed with the addition of bevacizumab to adjuvant FOLFOX. I found it particularly interesting that the rate of thrombocytopenia decreased among the patients who received bevacizumab (Allegra 2008; [1.2]).
![]() DR LOVE: Why do you think they did not see a significant increase in bowel perforations with bevacizumab in NSABP-C-08 (Allegra 2008; [1.2]) when it has been reported in the advanced-disease studies?
DR LOVE: Why do you think they did not see a significant increase in bowel perforations with bevacizumab in NSABP-C-08 (Allegra 2008; [1.2]) when it has been reported in the advanced-disease studies?
![]() DR HOFF: A couple of explanations are possible. One is that patients in the adjuvant trial were healthier than those with advanced disease. In the adjuvant setting, patients have less of a chance of having carcinomatosis, which could play a role in bowel perforation.
DR HOFF: A couple of explanations are possible. One is that patients in the adjuvant trial were healthier than those with advanced disease. In the adjuvant setting, patients have less of a chance of having carcinomatosis, which could play a role in bowel perforation.
Also, we cannot discard the possibility of numbers. One would not expect a large number of perforations in a trial the size of NSABP-C-08, with only a few more than 1,000 patients receiving bevacizumab.
![]() DR LOVE: They also did not report a significant increase in arterial events.
DR LOVE: They also did not report a significant increase in arterial events.
![]() DR HOFF: The trials are avoiding patients with a history of arterial events. Also, patients treated for cancer who are presumed free of disease tend to take good care of themselves in terms of diet and exercise. We must remember, however, that these data are preliminary. They are encouraging and may turn out to be true, but I believe it’s early for us to disregard the possibility of arterial events or bowel perforations in this population.
DR HOFF: The trials are avoiding patients with a history of arterial events. Also, patients treated for cancer who are presumed free of disease tend to take good care of themselves in terms of diet and exercise. We must remember, however, that these data are preliminary. They are encouraging and may turn out to be true, but I believe it’s early for us to disregard the possibility of arterial events or bowel perforations in this population.
![]() DR LOVE: Would you consider using bevacizumab in the adjuvant setting in your practice?
DR LOVE: Would you consider using bevacizumab in the adjuvant setting in your practice?
![]() DR HOFF: I believe it would be premature to use it to treat micrometastatic disease off study. At this point, no role exists in the adjuvant setting for these molecular-targeted agents — bevacizumab, cetuximab, panitumumab or any agents currently in the investigational phase. We should not forget the lesson we learned from irinotecan: We all expected it to be effective in the adjuvant setting, but it was not.
DR HOFF: I believe it would be premature to use it to treat micrometastatic disease off study. At this point, no role exists in the adjuvant setting for these molecular-targeted agents — bevacizumab, cetuximab, panitumumab or any agents currently in the investigational phase. We should not forget the lesson we learned from irinotecan: We all expected it to be effective in the adjuvant setting, but it was not.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2009 Research To Practice. All Rights Reserved. |