
 |
|||||||

| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-3
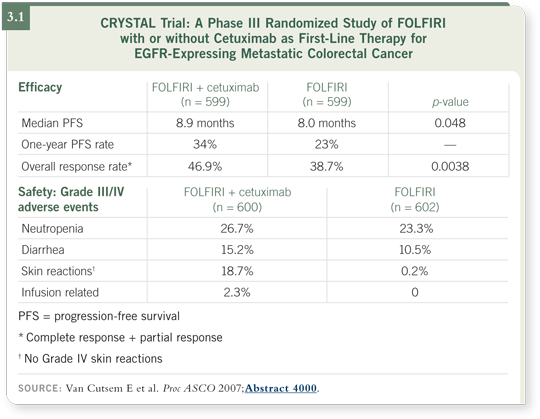
![]() DR LOVE: Would you discuss the reported data from the CRYSTAL trial,
including the K-ras data presented at ASCO?
DR LOVE: Would you discuss the reported data from the CRYSTAL trial,
including the K-ras data presented at ASCO?
![]() DR SALTZ: This was a 1,200-patient study investigating the addition of
cetuximab to FOLFIRI as first-line therapy — half of the patients received
FOLFIRI and half received FOLFIRI with cetuximab. The outcome of that
study was technically positive, but in my opinion it was disappointing (Van
Cutsem 2007; [3.1]).
DR SALTZ: This was a 1,200-patient study investigating the addition of
cetuximab to FOLFIRI as first-line therapy — half of the patients received
FOLFIRI and half received FOLFIRI with cetuximab. The outcome of that
study was technically positive, but in my opinion it was disappointing (Van
Cutsem 2007; [3.1]).
Progression-free survival, the specified primary endpoint, was improved in the overall study to a statistically significant degree, with a p-value of 0.048 (Van Cutsem 2007; [3.1]). The actual improvement in progression-free survival, however, was 27 days. I believe this raises the question of what is a clinically significant versus a statistically significant improvement.
I’m concerned that the skin toxicity associated with cetuximab has been underappreciated in terms of the significant impediment it imparts on quality of life. The rash can be painful or itchy, and the paronychial cracking, the paper-cut feeling in the fingers and toes, can become painful.
The CRYSTAL study demonstrated that progression-free survival is statistically significantly better with the addition of cetuximab to front-line therapy (Van Cutsem 2007; [3.1]). My interpretation, however, is that for the overall population, the incremental toxicity and probably the incremental costs are difficult to justify.
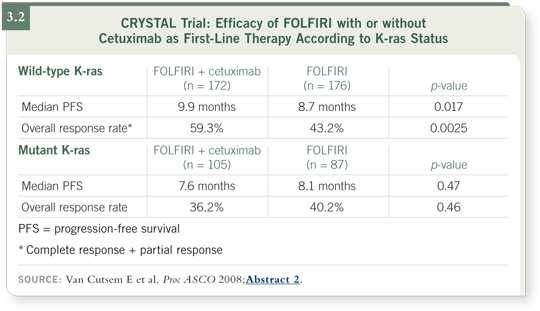
At ASCO, Dr Van Cutsem presented the results for patients for whom they had archived tissue and were able to evaluate K-ras mutation status. They studied the outcomes for the patients whose tumors had a wild-type K-ras gene versus those whose tumors had a mutant K-ras gene (Van Cutsem 2008).
The data suggest that for the patients whose tumors had a K-ras mutation, cetuximab added no value. The curves and outcomes data for those patients were the same with FOLFIRI as with FOLFIRI/cetuximab. The patients whose tumors had wild-type K-ras, approximately 60 to 65 percent of patients
in the study, derived more substantial benefit when cetuximab was added (Van Cutsem 2008; [3.2]).
These data can be interpreted in two ways. First, patients whose tumors have a K-ras mutation do not benefit from cetuximab and, therefore, ought not receive cetuximab in this scenario. Second, patients whose tumors have wild-type K-ras derive a greater degree of benefit from cetuximab (Van Cutsem 2008).
Instead of a 0.9-month progression-free survival benefit, it was a 1.2-month progression-free survival benefit. So now, instead of 27 days we’re up to 36 days. One must ask, should a median 36-day improvement in progression-free survival define a standard treatment? I don’t believe so.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |