
 |
|||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the EORTC trial 40983 evaluating perioperative
chemotherapy for resectable liver metastases?
DR LOVE: Would you discuss the EORTC trial 40983 evaluating perioperative
chemotherapy for resectable liver metastases?
![]() DR ALBERTS: This trial was designed to determine whether we could administer
three months of neoadjuvant chemotherapy to a patient with potentially
resectable hepatic metastases and not hinder the ability to perform the surgery,
either by causing liver toxicity or by allowing disease progression to a point at
which the tumor is no longer resectable. Then, if the surgery was successful,
three more months of chemotherapy was administered postoperatively, and
this regimen was compared to surgery alone.
DR ALBERTS: This trial was designed to determine whether we could administer
three months of neoadjuvant chemotherapy to a patient with potentially
resectable hepatic metastases and not hinder the ability to perform the surgery,
either by causing liver toxicity or by allowing disease progression to a point at
which the tumor is no longer resectable. Then, if the surgery was successful,
three more months of chemotherapy was administered postoperatively, and
this regimen was compared to surgery alone.
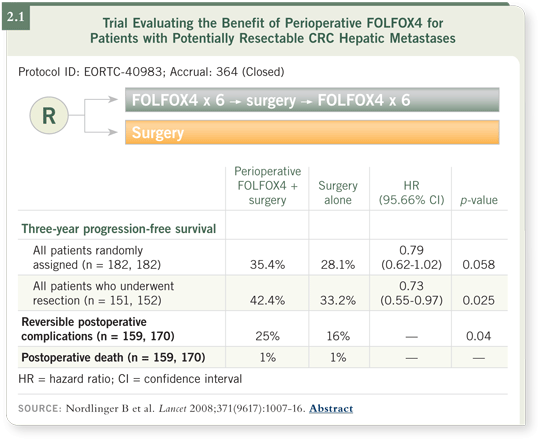
A statistically significant progression-free survival advantage was observed for the perioperative chemotherapy arm. However, a bar was set for a hazard ratio of 0.71 or less, which was not reached (Nordlinger 2008; [2.1]). With some reservations about the outcome, investigators are considering a trial of adjuvant therapy versus perioperative therapy, and the EORTC is evaluating the combination of biologic agents with the chemotherapy to try to enhance that perioperative outcome.
Another point to consider is that the patients enrolled in the trial were generally at good risk in that they typically had only one or two liver metastases. In that group — particularly patients with only one liver metastasis — the benefit of chemotherapy beyond surgery may be fairly small, depending on what agents the patient received in the past. Had we evaluated patients with three to five liver metastases, among whom the rate of recurrence is much higher, we might have seen a better outcome.
Track 2
![]() DR LOVE: What systemic strategies are being considered for converting
liver metastases from unresectable to resectable?
DR LOVE: What systemic strategies are being considered for converting
liver metastases from unresectable to resectable?
![]() DR ALBERTS: One approach is to try to maximize chemotherapy. Traditionally,
the trials have used either FOLFOX or FOLFIRI, but a recent European
trial for metastatic colorectal cancer evaluated “FOLFIRINOX” — the
combination of 5-FU, leucovorin, irinotecan and oxaliplatin. The secondary
endpoint in this trial was the rate of resection for patients with initially
unresectable, liver-only disease, and it demonstrated a much higher rate of
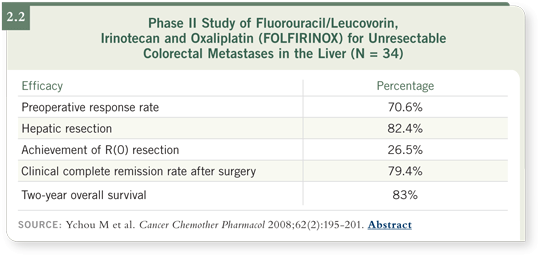
downsizing leading to surgery with FOLFIRINOX (Ychou 2008; [2.2]).
DR ALBERTS: One approach is to try to maximize chemotherapy. Traditionally,
the trials have used either FOLFOX or FOLFIRI, but a recent European
trial for metastatic colorectal cancer evaluated “FOLFIRINOX” — the
combination of 5-FU, leucovorin, irinotecan and oxaliplatin. The secondary
endpoint in this trial was the rate of resection for patients with initially
unresectable, liver-only disease, and it demonstrated a much higher rate of
downsizing leading to surgery with FOLFIRINOX (Ychou 2008; [2.2]).
Another strategy is to combine biologic agents with chemotherapy (Samalin 2008; [FOLFIRINOX with cetuximab]). Based on the recent K-ras data, we may need to screen patients: If the tumor has a wild-type K-ras expression, we would consider an EGFR inhibitor, such as cetuximab or panitumumab, and if K-ras is mutated, we would consider an agent such as bevacizumab.
Track 3
![]() DR LOVE: What is your approach when you are trying to convert a
potentially resectable tumor and the patient has received no previous
systemic therapy?
DR LOVE: What is your approach when you are trying to convert a
potentially resectable tumor and the patient has received no previous
systemic therapy?
![]() DR ALBERTS: One of the critical steps is to evaluate the patient in collaboration
with the surgeon. If the surgeon agrees that downsizing the tumor may
make it resectable, then the data, mainly from two prospective clinical trials,
suggest that FOLFOX and FOLFIRI are equivalent in terms of the response
rates in downsizing.
DR ALBERTS: One of the critical steps is to evaluate the patient in collaboration
with the surgeon. If the surgeon agrees that downsizing the tumor may
make it resectable, then the data, mainly from two prospective clinical trials,
suggest that FOLFOX and FOLFIRI are equivalent in terms of the response
rates in downsizing.
I believe most clinicians in the United States are more comfortable using FOLFOX. However, FOLFIRI is not any less efficacious in this setting. Probably the best option for downsizing the tumor rapidly would be FOLFOX and a biologic agent. Some data with disease treatment in the metastatic setting at any site, not only for colorectal liver-only metastases, suggest that combining cetuximab with FOLFIRI or FOLFOX increases the response rate (Tabernero 2007; Ciuleanu 2008).
Obviously we need to take into account the K-ras status of the tumor. In my practice, if a patient is chemotherapy naïve, I use either FOLFOX or FOLFIRI — preferably FOLFOX — combined with cetuximab. If the tumor has a K-ras mutation, then I typically add bevacizumab.
![]() DR LOVE: What is your first-line therapy for metastatic disease in clinical
practice?
DR LOVE: What is your first-line therapy for metastatic disease in clinical
practice?
![]() DR ALBERTS: Generally I recommend chemotherapy with bevacizumab.
However, with the evolving data on K-ras and evidence from two large
European studies, the CRYSTAL (Van Cutsem 2008) and OPUS (Bokemeyer
2008) trials, which evaluated FOLFIRI or FOLFOX with cetuximab, we have
a good rationale for using cetuximab in the front-line setting, and I have done
so recently.
DR ALBERTS: Generally I recommend chemotherapy with bevacizumab.
However, with the evolving data on K-ras and evidence from two large
European studies, the CRYSTAL (Van Cutsem 2008) and OPUS (Bokemeyer
2008) trials, which evaluated FOLFIRI or FOLFOX with cetuximab, we have
a good rationale for using cetuximab in the front-line setting, and I have done
so recently.
Tracks 9-10
![]() DR LOVE: What are some of the research strategies now being tested in
the adjuvant setting?
DR LOVE: What are some of the research strategies now being tested in
the adjuvant setting?
![]() DR ALBERTS: We are awaiting the results of NSABP-C-08, evaluating
FOLFOX with or without bevacizumab. Data from this trial may be available
in 2009 or 2010. If bevacizumab demonstrates a benefit, then FOLFOX with
bevacizumab will likely become the new standard. The question then would
be how to build on this trial.
DR ALBERTS: We are awaiting the results of NSABP-C-08, evaluating
FOLFOX with or without bevacizumab. Data from this trial may be available
in 2009 or 2010. If bevacizumab demonstrates a benefit, then FOLFOX with
bevacizumab will likely become the new standard. The question then would
be how to build on this trial.
The other approach under investigation is FOLFOX with or without cetuximab in the NCCTG-N0147 trial. That trial is still accruing patients, and we may not have data for another three years. In the meantime, I don’t sense a firm direction.
![]() DR LOVE: Would you summarize the safety data that were presented at the
last ASCO meeting from the NSABP-C-08 trial?
DR LOVE: Would you summarize the safety data that were presented at the
last ASCO meeting from the NSABP-C-08 trial?
![]() DR ALBERTS: Patients on the FOLFOX with bevacizumab arm received this
regimen for a full year, so concern was raised about toxicities, such as bleeding
and bowel perforation. However, the safety data revealed no significant
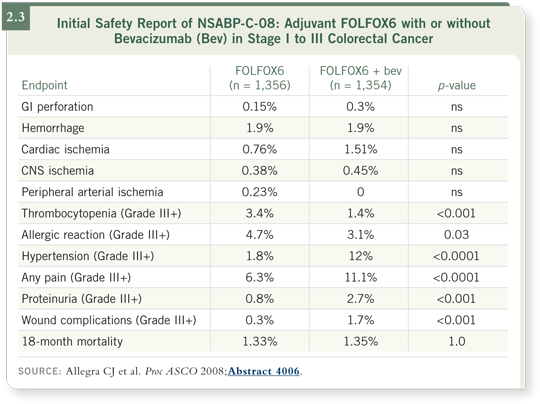
increase in these side effects with bevacizumab (Allegra 2008; [2.3]).
DR ALBERTS: Patients on the FOLFOX with bevacizumab arm received this
regimen for a full year, so concern was raised about toxicities, such as bleeding
and bowel perforation. However, the safety data revealed no significant
increase in these side effects with bevacizumab (Allegra 2008; [2.3]).
In terms of bowel perforations, one thought is that perhaps in the advanced setting, metastatic disease is attached to the bowel wall and that, with a rapid regression, somehow leads to a perforation. Obviously, everybody is pleased about these data, and if C-08 turns out to be a positive trial, we know it is safe to use this approach.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |