
 |
|||||||
| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: What were your thoughts as you saw the clinical data evolve
in the field of anti-angiogenesis, particularly the observation that in colon,
breast and lung cancer bevacizumab seemed to work better with chemotherapy?
DR LOVE: What were your thoughts as you saw the clinical data evolve
in the field of anti-angiogenesis, particularly the observation that in colon,
breast and lung cancer bevacizumab seemed to work better with chemotherapy?
![]() DR JAIN: In 1996, we naïvely believed that we could destroy the tumor
vasculature by blocking VEGF with bevacizumab. We learned that bevacizumab
reduced blood vessel density, but then there was a second wave of
angiogenesis, which resulted in tumor relapse. This suggested that we needed
to get rid of the source of the angiogenic molecules — VEGF, FGF, IL6, IL8,
et cetera — which meant killing cells with chemotherapy or radiation therapy.
DR JAIN: In 1996, we naïvely believed that we could destroy the tumor
vasculature by blocking VEGF with bevacizumab. We learned that bevacizumab
reduced blood vessel density, but then there was a second wave of
angiogenesis, which resulted in tumor relapse. This suggested that we needed
to get rid of the source of the angiogenic molecules — VEGF, FGF, IL6, IL8,
et cetera — which meant killing cells with chemotherapy or radiation therapy.
Parallel to our preclinical work, clinical reports were being published that demonstrated that combining anti-VEGF therapy with chemotherapy was effective. This was paradoxical because blood vessels are necessary to deliver chemotherapy and the oxygen necessary for radiation therapy. How could we destroy the vasculature with agents such as bevacizumab and expect better outcomes?
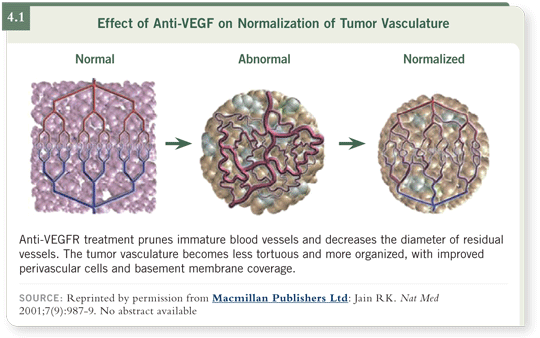
These clinical observations led to the hypothesis that perhaps we were destroying some vessels and repairing the remainder so they become more normal. I published the normalization hypothesis in a commentary in Nature Medicine in 2001 (Jain 2001). We had preclinical evidence in 1998 that if testosterone is removed from testosterone-dependent tumors, VEGF is lowered in a similar manner as with bevacizumab and the blood vessels became straighter, narrower and more normal (4.1). We observed the same phenomenon with trastuzumab, except that it worked not by lowering VEGF but by upregulating thrombospondin, which is an endogenous anti-angiogenic molecule. That’s when I realized that the abnormal vasculature resulted from an abundance of proangiogenic molecules and a paucity of anti-angiogenic molecules.
Tracks 4-5, 7
![]() DR LOVE: Would you discuss the trial you were involved with evaluating
chemoradiation therapy with bevacizumab for patients with rectal cancer,
which was presented at ASCO this year?
DR LOVE: Would you discuss the trial you were involved with evaluating
chemoradiation therapy with bevacizumab for patients with rectal cancer,
which was presented at ASCO this year?
![]() DR JAIN: The protocol was a Phase I/II dose-escalation trial. The initial dose
of bevacizumab was five milligrams per kilogram. The trial design was such
that we could view the effect on tumor biology of bevacizumab alone or in
combination with chemotherapy.
DR JAIN: The protocol was a Phase I/II dose-escalation trial. The initial dose
of bevacizumab was five milligrams per kilogram. The trial design was such
that we could view the effect on tumor biology of bevacizumab alone or in
combination with chemotherapy.
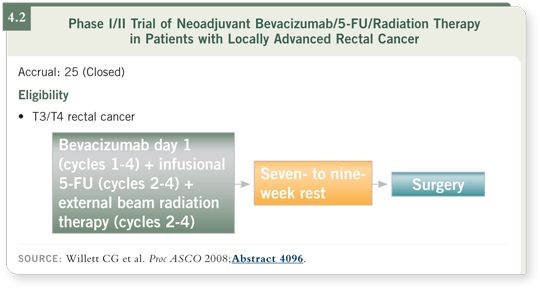
I do not know of any other trial in which so much information has been obtained from so few patients. The patients received bevacizumab initially by itself, followed by chemotherapy, radiation therapy and bevacizumab. Then a rest of seven to nine weeks was followed by surgical resection (Willett 2008; [4.2]). The results were presented at ASCO 2008. We have to be cautious in interpreting the data because it was not a randomized trial. Three-year overall survival and three-year local control rates were 100 percent, and disease-free survival was approximately 90 percent (Willett 2008).
I believe as soon as our work is written up and others can see the results of this Phase II trial, it calls for a randomized Phase III trial. We have plenty of evidence that adding radiation therapy to this neoadjuvant treatment of bevacizumab combined with chemotherapy is helping patients.
![]() DR LOVE: At a basic level, I guess the idea would be that you’re increasing
the normal distribution of blood to allow radiation therapy to function more
effectively?
DR LOVE: At a basic level, I guess the idea would be that you’re increasing
the normal distribution of blood to allow radiation therapy to function more
effectively?
![]() DR JAIN: Yes, the key rationale is to improve oxygenation. You don’t need to
increase blood flow. You simply have to distribute it more uniformly because
the blood flow is heterogeneous in tumors. Some regions receive decent
amounts of oxygen, and other regions are hypoxic. Those hypoxic regions,
unfortunately, can harbor tumor cells that can lead to relapse.
DR JAIN: Yes, the key rationale is to improve oxygenation. You don’t need to
increase blood flow. You simply have to distribute it more uniformly because
the blood flow is heterogeneous in tumors. Some regions receive decent
amounts of oxygen, and other regions are hypoxic. Those hypoxic regions,
unfortunately, can harbor tumor cells that can lead to relapse.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |