
| Tracks 1-11 |
| Track 1 |
Introduction |
| Track 2 |
US BRiTE registry trial: Side
effects and toxicity of bevacizumab
in clinical practice |
| Track 3 |
Predicting risk of bevacizumab-associated
arterial thrombotic
events |
| Track 4 |
Acquired hypertension as a
predictor of response to bevacizumab |
| Track 5 |
Cetuximab-associated rash:
Implications for adjuvant therapy |
| Track 6 |
Preoperative versus postoperative
chemoradiation therapy for rectal
cancer |
|
| Track 7 |
The importance of downstaging
rectal cancer |
| Track 8 |
Rationale for incorporating
oxaliplatin with preoperative
radiation therapy for rectal cancer |
| Track 9 |
Selection of oral versus infusional
fluoropyrimidine therapy |
| Track 10 |
Geographic variability in the
tolerability of fluoropyrimidines |
| Track 11 |
Use of preoperative response
to chemoradiation therapy to
determine postoperative adjuvant
therapy for rectal cancer |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: Can you discuss the findings of the US BRiTE registry trial?
 DR HALLER: I believe American oncologists are doing a wonderful job of
selecting patients. The BRiTE registry showed that the toxicity profile of
bevacizumab for the average patient in the community is similar to those in
clinical trials. This is important information to have. Sometimes we worry
that patients in trials are so extraordinarily selected that toxicity findings will
not apply to our patients.
DR HALLER: I believe American oncologists are doing a wonderful job of
selecting patients. The BRiTE registry showed that the toxicity profile of
bevacizumab for the average patient in the community is similar to those in
clinical trials. This is important information to have. Sometimes we worry
that patients in trials are so extraordinarily selected that toxicity findings will
not apply to our patients.
We know that 20 percent of patients with metastatic disease should not receive
bevacizumab because of the high risk of arterial thrombotic events, perforation
or other adverse events. I believe 80 percent might be the correct number of
patients to be receiving bevacizumab.
 DR LOVE: Where are we in terms of understanding the side effects and
toxicity of bevacizumab?
DR LOVE: Where are we in terms of understanding the side effects and
toxicity of bevacizumab?
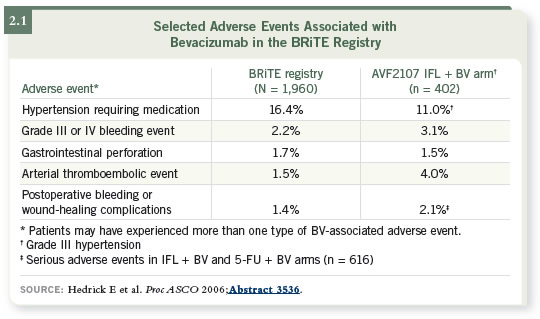
 DR HALLER: We know that the toxicity profile we saw in the AVF2107 trial
is somewhat predictive of what we see in clinical practice (Hurwitz 2003;
Hedrick 2006; [2.1]). As a practitioner, that is comforting — I can tell a
patient what to expect.
DR HALLER: We know that the toxicity profile we saw in the AVF2107 trial
is somewhat predictive of what we see in clinical practice (Hurwitz 2003;
Hedrick 2006; [2.1]). As a practitioner, that is comforting — I can tell a
patient what to expect.
Track 8
 DR LOVE:
DR LOVE: The NSABP-R-04 trial, which originally compared preoperative
capecitabine and radiation therapy to continuous infusion 5-FU and
radiation therapy, has now been amended to include oxaliplatin. What do
we know about neoadjuvant oxaliplatin?
 DR HALLER: Our preclinical rationale is something we have inferred from
laboratory evidence — that platinates will be synergistic with radiation
therapy. This is why platinums and fluoropyrimidines have been used in head
and neck cancer, lung cancer, cervical cancer and other settings.
DR HALLER: Our preclinical rationale is something we have inferred from
laboratory evidence — that platinates will be synergistic with radiation
therapy. This is why platinums and fluoropyrimidines have been used in head
and neck cancer, lung cancer, cervical cancer and other settings.
Our clinical rationale stems from two US trials, CALGB 89901 and ECOG
1297 (Ryan 2006; Rosenthal 2003). These trials incorporated infusional 5-FU
and either biweekly or weekly oxaliplatin and showed pathologic complete
response rates in the mid 20 percent range, compared to about 10 percent in
other trials, including the German study with preoperative 5-FU and radiation
therapy (Sauer 2004).
Indeed, across a series of Phase I-II trials, we see reliable pathologic complete
response rates from the midteens up to about 40 percent. To me, the consistency
of the data is important.
At our institution, we collected data from a large series of patients who were
not enrolling in a study but received preoperative 5-FU with or without oxaliplatin.
When we examined these cases retrospectively, we saw that the pathologic complete response rates were equivalent to those seen in the ECOG trial (Dolinsky 2006).
The data were so intriguing to our radiation oncologists and surgeons that
they hesitated to refer a patient unless they believed the patient might receive
oxaliplatin as part of a “standard” regimen.
Track 10
 DR LOVE:
DR LOVE: Can you summarize the fascinating and long-awaited data
that you presented at ASCO on the side effects of fluoropyrimidines and
geography?
 DR HALLER: As American oncologists, we all thought we were doing
something wrong. What was it about our patients that made them appear to
suffer so much more toxicity from capecitabine than the patients described in
trials? Dr Hans Schmoll and I co-chaired a study that compared CAPOX with
either the Roswell or the Mayo Clinic 5-FU regimens in 1,800 patients with
Stage III colon cancer (Schmoll 2005). When we evaluated toxicity differences
by region, we saw on the surface that the US population clearly had more
toxicity.
DR HALLER: As American oncologists, we all thought we were doing
something wrong. What was it about our patients that made them appear to
suffer so much more toxicity from capecitabine than the patients described in
trials? Dr Hans Schmoll and I co-chaired a study that compared CAPOX with
either the Roswell or the Mayo Clinic 5-FU regimens in 1,800 patients with
Stage III colon cancer (Schmoll 2005). When we evaluated toxicity differences
by region, we saw on the surface that the US population clearly had more
toxicity.
When we combined our data with two trials of the Mayo Clinic regimen
versus capecitabine in advanced disease (Hoff 2001; van Cutsem 2001), the
toxicity profile was consistent across the board. We then reviewed historically
the IMPACT data (IMPACT 1995), which were a compilation of 5-FU
and leucovorin regimens versus surgery alone in colon cancer, and saw that
Europeans had less toxicity with any 5-FU and leucovorin regimen.
At ASCO this year Dr Schmoll presented toxicity data for the CAPOX study
(Schmoll 2006), and I presented a poster on capecitabine in both the metastatic
and adjuvant settings with or without oxaliplatin (Haller 2006).
For US patients versus non-US patients, the hazard ratios were about 1.8 for
almost any toxicity you could name, including myelosuppression, a nonself-reported
toxicity, and some self-reported toxicities such as diarrhea and
mucositis (2.2).
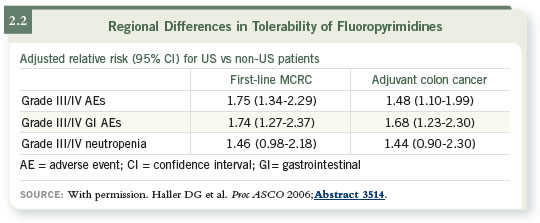
So it is no longer unclear whether Americans have more toxicity — it is now a
known truth. Possible explanations for these differences include differences in
pharmacogenetic factors or differences in external environmental factors, such
as diet. Dr Carmen Allegra has shown that certain foods or supplements — and
American diets are very high in folates — may be contributing to toxicity.
Select publications

