
| Tracks 1-15 |
| Track 1 |
Introduction |
| Track 2 |
OPTIMOX2: Maintenance therapy
or chemotherapy-free intervals
after FOLFOX |
| Track 3 |
Clinical trial incorporating a
maintenance or chemotherapy-free
interval strategy with bevacizumab |
| Track 4 |
Selection of FOLFOX or FOLFIRI
with bevacizumab as first-line
therapy |
| Track 5 |
Therapeutic approach with
curative intent for patients with
metastatic disease |
| Track 6 |
Treatment of patients with
synchronous primary and
metastatic disease |
| Track 7 |
Clinical management of
potentially resectable hepatic-only
metastases |
| Track 8 |
NSABP-C-09: CAPOX with or
without hepatic arterial infusion of
floxuridine for resected or ablated
liver metastases |
|
| Track 9 |
Ongoing adjuvant clinical trials
evaluating FOLFOX with biologic
therapy |
| Track 10 |
Tolerability and side effects of
biologic therapies in the adjuvant
setting |
| Track 11 |
Potential rationale for efficacy of
adjuvant bevacizumab |
| Track 12 |
Selection of adjuvant chemotherapeutic
regimens |
| Track 13 |
Use of adjuvant fluoropyrimidine
monotherapy |
| Track 14 |
NSABP-R-04: Preoperative
radiation therapy and oral versus
intravenous 5-FU with or without
oxaliplatin for rectal cancer |
| Track 15 |
Use of postoperative adjuvant
therapy for rectal cancer |
|
|
Select Excerpts from the Interview
Track 3
 DR LOVE:
DR LOVE: Can you discuss the OPTIMOX data sets presented at ASCO?
 DR GROTHEY: I believe the most important trial in this regard was the
OPTIMOX2 trial (Maindrault-Goebel 2006), which was based on a prior trial
conducted in France, the OPTIMOX1 trial (Tournigand 2006). OPTIMOX1
was a Phase III trial comparing FOLFOX4 continued until patients either developed toxicities or their disease progressed to a stop-and-go regimen — with the
stop-and-go related to oxaliplatin — meaning that patients were treated with a
limited duration of FOLFOX then continued on a maintenance therapy of 5-FU
alone, and oxaliplatin was to be reintroduced at a planned interval.
DR GROTHEY: I believe the most important trial in this regard was the
OPTIMOX2 trial (Maindrault-Goebel 2006), which was based on a prior trial
conducted in France, the OPTIMOX1 trial (Tournigand 2006). OPTIMOX1
was a Phase III trial comparing FOLFOX4 continued until patients either developed toxicities or their disease progressed to a stop-and-go regimen — with the
stop-and-go related to oxaliplatin — meaning that patients were treated with a
limited duration of FOLFOX then continued on a maintenance therapy of 5-FU
alone, and oxaliplatin was to be reintroduced at a planned interval.
OPTIMOX2 went one step further by introducing the complete chemotherapy-
free interval — giving patients a complete break from all treatment
until their disease progressed and comparing it to maintenance therapy.
The problem with this trial for our current practice, beyond the fact that it
did not include bevacizumab, was that this trial design allowed tumors to
grow back to their initial size. Patients who had a response to FOLFOX-based
therapy and discontinued FOLFOX after three months of therapy were then
followed and tumors were allowed to progress back to their initial presentation.
I believe this would currently be quite a hard sell to patients.
Within these limitations, and based on the fact that only 200 patients were
assigned in this trial, no significant difference appeared in the duration of
disease control as defined by the study. We don’t have any data on overall
survival yet, and we don’t have quality-of-life data, but at least withholding
therapy did not appear to harm patients.
Track 4
 DR LOVE:
DR LOVE: How do you make the decision between FOLFOX and
FOLFIRI to combine with bevacizumab in the first-line setting?
 DR GROTHEY: If we are treating in the neoadjuvant potentially curative
setting, an abundance of data support FOLFOX over FOLFIRI and oxaliplatin
over irinotecan, particularly the recent data coming from MD Anderson about
the effects of liver toxicity on therapy and postoperative, postliver resection
mortality or morbidity (Vauthey 2006).
DR GROTHEY: If we are treating in the neoadjuvant potentially curative
setting, an abundance of data support FOLFOX over FOLFIRI and oxaliplatin
over irinotecan, particularly the recent data coming from MD Anderson about
the effects of liver toxicity on therapy and postoperative, postliver resection
mortality or morbidity (Vauthey 2006).
Outside of that, I believe whether you use FOLFOX or FOLFIRI is “a wash.”
Outside of a clinical trial, I talk to my patients about which toxicity they
would prefer. It’s either the neurotoxicity or the higher risk of developing
early onset diarrhea. From an efficacy point of view, FOLFOX and FOLFIRI
are not different in the palliative setting.
 DR LOVE: We don’t have direct comparisons of FOLFOX/bevacizumab
versus FOLFIRI/bevacizumab, but can you make any indirect conclusions
from the BICC-C trial data and the TREE trial data?
DR LOVE: We don’t have direct comparisons of FOLFOX/bevacizumab
versus FOLFIRI/bevacizumab, but can you make any indirect conclusions
from the BICC-C trial data and the TREE trial data?
 DR GROTHEY: Yes. The TREE trials (Hochster 2006, 2005) and the BICC-C
trial (Fuchs 2006) were similar. The TREE-1 and BICC-C trials both started
the year before bevacizumab was approved, so both trials did not include
bevacizumab in their first phase, and both trials evaluated what is the best
fluoropyrimidine, in combination with either oxaliplatin in the TREE trials
or irinotecan in the BICC-C trial.
DR GROTHEY: Yes. The TREE trials (Hochster 2006, 2005) and the BICC-C
trial (Fuchs 2006) were similar. The TREE-1 and BICC-C trials both started
the year before bevacizumab was approved, so both trials did not include
bevacizumab in their first phase, and both trials evaluated what is the best
fluoropyrimidine, in combination with either oxaliplatin in the TREE trials
or irinotecan in the BICC-C trial.
After bevacizumab was approved in the United States, the TREE trial,
which evaluated oxaliplatin combinations, was amended to include bevacizumab
in all three treatment arms. The capecitabine dose in arm three was
reduced to account for toxicities observed in TREE-1, and the BICC-C trial
discontinued the capecitabine and irinotecan arm and added bevacizumab to
FOLFIRI and IFL.
What we have now is a cross-trial comparison. When you compare FOLFOX
and FOLFIRI with bevacizumab in TREE-2 and in the BICC-C trial, it’s
interesting to see that the progression-free survival in both trials — a crosstrial
comparison for FOLFOX with bevacizumab and FOLFIRI with bevacizumab
— was 9.9 months, exactly identical. The response rates were 54 to 55
percent, almost identical (1.1).
We have data on FOLFOX with bevacizumab in terms of overall survival,
which was 26 months in TREE-2. For FOLFIRI with bevacizumab, the
endpoint for overall survival in the BICC-C Phase II trial had not yet been
reached, but the survival curve suggested that it will be beyond two years.
So we have similar data on FOLFIRI and FOLFOX using almost all efficacy
parameters.

Track 9
 DR LOVE:
DR LOVE: How are you approaching patients in the adjuvant setting in
terms of nonprotocol therapy?
 DR GROTHEY: Recently we have moved from 5-FU to FOLFOX as standard
of care in the adjuvant setting for colon cancer and, for most purposes, also
in rectal cancer. So the new question is how to integrate the biologic agents
that we know work in the palliative setting — cetuximab and bevacizumab.
Both of these biologics have demonstrated efficacy in established colorectal
cancer tumors, but it has not been determined whether they play any role in
the adjuvant setting.
DR GROTHEY: Recently we have moved from 5-FU to FOLFOX as standard
of care in the adjuvant setting for colon cancer and, for most purposes, also
in rectal cancer. So the new question is how to integrate the biologic agents
that we know work in the palliative setting — cetuximab and bevacizumab.
Both of these biologics have demonstrated efficacy in established colorectal
cancer tumors, but it has not been determined whether they play any role in
the adjuvant setting.
We can’t immediately transfer all our knowledge and our experience from
the palliative setting into the adjuvant setting. This was demonstrated by the
relative failure of irinotecan to show benefit in the adjuvant setting (Saltz
2004), whereas in the palliative setting FOLFOX and FOLFIRI are equivalent.
Cetuximab is an EGFR antibody, and bevacizumab is a VEGF inhibitor.
Both are being tested in the United States in ongoing cooperative group
trials. NSABP-C-08 is evaluating FOLFOX with or without bevacizumab as
adjuvant therapy for Stage II and III colon cancer. NCCTG-N0147 is evaluating
FOLFOX with or without cetuximab in the adjuvant setting.
ECOG has a Stage II trial (E5202) observing patients at molecular high risk
as defined by microsatellite instability and loss of heterozygosity at the 18q
chromosome. Those patients will be randomly assigned to FOLFOX with or
without bevacizumab — analogous to the C-08 trial.
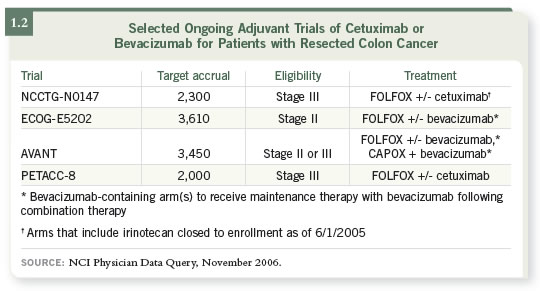
For every patient that we see right now in the adjuvant setting, Stage II
and Stage III, there is an adjuvant trial available (1.2). These trials are being
mirrored by European trials that are similar in design: The AVANT trial is
evaluating bevacizumab, and the PETACC trial is evaluating cetuximab.
Track 14
 DR LOVE:
DR LOVE: In rectal cancer, the NSABP is evaluating the use of
capecitabine versus continuous infusion 5-FU with or without oxaliplatin.
What do we know about both of these questions?
 DR GROTHEY: The NSABP-R-04 trial was initially designed simply to
compare capecitabine to continuous infusion 5-FU, but over time this
question became more and more secondary. We want to optimize not only
local control but also the systemic effects of therapy so that micrometastases
can be treated as early as possible. So evaluating an oxaliplatin-based combination
made sense because we also know that oxaliplatin is a good radiosensitizer
and has systemic efficacy at a dose of 50 mg/m2 on a weekly basis.
DR GROTHEY: The NSABP-R-04 trial was initially designed simply to
compare capecitabine to continuous infusion 5-FU, but over time this
question became more and more secondary. We want to optimize not only
local control but also the systemic effects of therapy so that micrometastases
can be treated as early as possible. So evaluating an oxaliplatin-based combination
made sense because we also know that oxaliplatin is a good radiosensitizer
and has systemic efficacy at a dose of 50 mg/m2 on a weekly basis.
The standard of care right now outside of a clinical trial would be to use
continuous infusion 5-FU. A growing body of evidence suggests that capecitabine at a dose of 825 mg/m2 BID from Monday to Friday with
weekend breaks throughout radiation can be safely administered, and this
regimen has similar efficacies in Phase II cross-trial comparisons.
Select publications

