You are here: Home:
CCU 5 | 2006: Charles S Fuchs, MD, MPH

| Tracks 1-13 |
| Track 1 |
Introduction |
| Track 2 |
Use of irinotecan-containing
regimens in the adjuvant setting |
| Track 3 |
Effects of physical activity on
patients with colon cancer |
| Track 4 |
Influence of regular aspirin use
on survival for patients with colon
cancer |
| Track 5 |
Potential mechanisms of aspirin,
diet and exercise on risk of
cancer recurrence |
| Track 6 |
Feasibility of prospectively
evaluating aspirin, diet or exercise
in clinical trials |
| Track 7 |
Counseling patients about dietary
and lifestyle modifications |
|
| Track 8 |
Selection of adjuvant
chemotherapy for patients with
Stage II disease |
| Track 9 |
Ongoing adjuvant clinical trials
incorporating biologic therapies |
| Track 10 |
Studies evaluating chemotherapy
in combination with biologic
doublets in the metastatic setting |
| Track 11 |
Approach to patients with
synchronous primary and
metastatic colon cancer |
| Track 12 |
Selection of first-line
chemotherapy |
| Track 13 |
OPTIMOX2: Maintenance therapy
or chemotherapy-free intervals
after FOLFOX for patients with
metastatic disease |
|
|
Select Excerpts from the Interview
Track 3
 DR LOVE:
DR LOVE: Can you review your data evaluating exercise in the adjuvant
trial of IFL (CALGB-C89803)?
 DR FUCHS: In CALGB-C89803, which was a negative trial in terms of the
primary endpoint (Saltz 2004), we provided patients with a 16-page questionnaire
about diet and lifestyle. Patients completed the questionnaire, which
was validated through various studies such as the Nurses’ Health Study. In
the CALGB trial, the surveys were administered midway through adjuvant
therapy and about six months after its completion (Meyerhardt 2006a, b).
DR FUCHS: In CALGB-C89803, which was a negative trial in terms of the
primary endpoint (Saltz 2004), we provided patients with a 16-page questionnaire
about diet and lifestyle. Patients completed the questionnaire, which
was validated through various studies such as the Nurses’ Health Study. In
the CALGB trial, the surveys were administered midway through adjuvant
therapy and about six months after its completion (Meyerhardt 2006a, b).
Compliance with the questionnaire was excellent — about 95 percent of the
patients completed it. Physical activity was highly protective and associated
with a significant improvement in disease-free survival. The more physically active the patient, the better the disease-free and overall survival (Meyerhardt
2006a).
 DR LOVE: Did the physical activity occur during chemotherapy?
DR LOVE: Did the physical activity occur during chemotherapy?
 DR FUCHS: It was during and after chemotherapy. We measured it at two
time points. If the patients walked an average of about six hours per week,
they had a 47 percent improvement in disease-free survival (Meyerhardt
2006a).
DR FUCHS: It was during and after chemotherapy. We measured it at two
time points. If the patients walked an average of about six hours per week,
they had a 47 percent improvement in disease-free survival (Meyerhardt
2006a).
One might argue that the physically active patients were healthier and
the patients who were inactive had occult cancer. We considered that and
repeated the analysis by excluding all of the events in the first six months after
completing the questionnaire, and we found the same results (Meyerhardt
2006a). We also repeated the analysis for the patients with colon cancer in
the Nurses’ Health Study who had completed the same questionnaire, and the
findings were identical (Meyerhardt 2006b).
Track 8
 DR LOVE:
DR LOVE: How do you approach the decision about adjuvant chemotherapy
off protocol, particularly for patients with Stage II disease?
 DR FUCHS: I use the clinical features we are familiar with, such as perforation
and obstruction and the number of lymph nodes sampled. I try to pool
together patients I don’t believe would benefit from adjuvant therapy, those
with whom I’m comfortable using a f luoropyrimidine alone and those for
whom I would use FOLFOX.
DR FUCHS: I use the clinical features we are familiar with, such as perforation
and obstruction and the number of lymph nodes sampled. I try to pool
together patients I don’t believe would benefit from adjuvant therapy, those
with whom I’m comfortable using a f luoropyrimidine alone and those for
whom I would use FOLFOX.
In patients with higher-risk disease — those with few lymph nodes analyzed
or those with adherence to or invasion of adjacent structures who might have
obstruction or perforation — I’m comfortable using FOLFOX. I have to
admit, however, that I’m still willing to use f luoropyrimidine monotherapy
for patients with more standard-risk disease, although I know some of my
colleagues routinely use FOLFOX in all circumstances.
Although the proportional benefit of FOLFOX is fairly consistent across
patients with Stage II or Stage III disease, I also want to consider the absolute
benefits, in a particularly low-risk setting. Is the addition of oxaliplatin, with
its inherent neuropathy risk, necessary in patients for whom the absolute
benefit is not so great?
 DR LOVE: When you are going to use f luoropyrimidine monotherapy, do you
bring up the possibility of capecitabine?
DR LOVE: When you are going to use f luoropyrimidine monotherapy, do you
bring up the possibility of capecitabine?
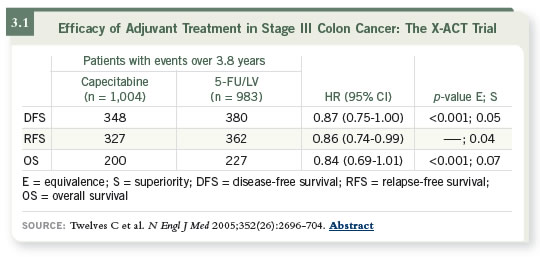
 DR FUCHS: I do. The X-ACT study is a compelling effort (Twelves 2005;
[3.1]), although the majority of the patients were enrolled in Europe. Dan
Haller has clearly demonstrated that the toxicity associated with capecitabine
differs on each side of the Atlantic (Haller 2006). Although you can use 2,500
mg/m2 per day in Europe with reasonable tolerability, it is difficult to use those
doses in the United States. The quandary is that this is an adjuvant setting and we want to use the standard dose used in the X-ACT trial, yet most patients in
North America don’t tolerate that dose.
DR FUCHS: I do. The X-ACT study is a compelling effort (Twelves 2005;
[3.1]), although the majority of the patients were enrolled in Europe. Dan
Haller has clearly demonstrated that the toxicity associated with capecitabine
differs on each side of the Atlantic (Haller 2006). Although you can use 2,500
mg/m2 per day in Europe with reasonable tolerability, it is difficult to use those
doses in the United States. The quandary is that this is an adjuvant setting and we want to use the standard dose used in the X-ACT trial, yet most patients in
North America don’t tolerate that dose.
Track 11
 DR LOVE:
DR LOVE: How do you approach patients who present with synchronous
primary and metastatic colon cancer?
 DR FUCHS: I consider the possibility of not sending them to up-front surgery.
If the tumor is on the right side, where the risk of obstruction is reasonably
low, if they don’t demonstrate any obstructive symptoms and if there isn’t any
obvious bleeding and I’m not concerned about perforation, sending them for a
resection would just delay the start of systemic therapy.
DR FUCHS: I consider the possibility of not sending them to up-front surgery.
If the tumor is on the right side, where the risk of obstruction is reasonably
low, if they don’t demonstrate any obstructive symptoms and if there isn’t any
obvious bleeding and I’m not concerned about perforation, sending them for a
resection would just delay the start of systemic therapy.
I would start them on a regimen of chemotherapy with bevacizumab without
sending them for a resection. Some are concerned about the possibility that
perforation might occur if the primary is in place, but those data have not
been borne out.
Select publications

