
| Tracks 1-15 |
| Track 1 |
Introduction |
| Track 2 |
Recent trends in colorectal
cancer clinical research |
| Track 3 |
Combining bevacizumab/
cetuximab plus chemotherapy |
| Track 4 |
Multimodality therapeutic
approach to patients with
hepatic-only disease |
| Track 5 |
Role of aggressive surveillance
in earlier detection of recurrent
disease |
| Track 6 |
Adjuvant therapy for patients with
Stage II/III colon cancer |
| Track 7 |
Clinical implications of the
X-ACT adjuvant trial comparing
capecitabine to 5-FU/LV |
| Track 8 |
Dosing capecitabine in the
adjuvant setting |
|
| Track 9 |
Substitution of capecitabine for
5-FU in combination with
oxaliplatin |
| Track 10 |
Current generation of adjuvant
trials in colon cancer |
| Track 11 |
Potential side effects of biologic
agents in the adjuvant setting |
| Track 12 |
Oxaliplatin-associated
neurotoxicity |
| Track 13 |
Advances in the treatment of
rectal cancer |
| Track 14 |
Incorporation of capecitabine and
oxaliplatin into neoadjuvant trials
in rectal cancer |
| Track 15 |
Clinical and research developments
in colorectal cancer
presented at ASCO 2006 |
|
|
Select Excerpts from the Interview
Track 6
 DR LOVE:
DR LOVE: Let’s talk about adjuvant therapy of colon cancer. Can you
discuss your approach to off-protocol treatment and where you think
we’re heading in terms of the next generation of clinical trials?
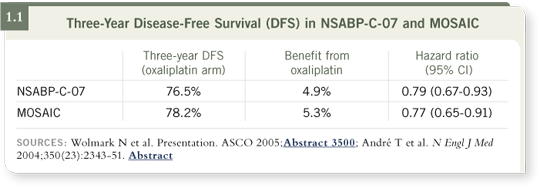
 DR ROTHENBERG: The MOSAIC trial from Europe (André 2004) and the
C-07 trial from the NSABP (Wolmark 2005) both demonstrated that the
addition of oxaliplatin to 5-FU/leucovorin improved three-year progression-free survival by approximately four to five percentage points, which was
significant in both trials (1.1).
DR ROTHENBERG: The MOSAIC trial from Europe (André 2004) and the
C-07 trial from the NSABP (Wolmark 2005) both demonstrated that the
addition of oxaliplatin to 5-FU/leucovorin improved three-year progression-free survival by approximately four to five percentage points, which was
significant in both trials (1.1).
We have to be concerned about long-term toxicity with these kinds of
regimens. If we counsel patients about potential permanent but mild neuropathy
and they’re accepting of this, then we have no reason not to administer
oxaliplatin/5-FU/leucovorin — whether we use the schedule that the NSABP
used, on which a lower total dose of oxaliplatin was administered, or the every
two-week infusional 5-FU regimen from the MOSAIC trial.
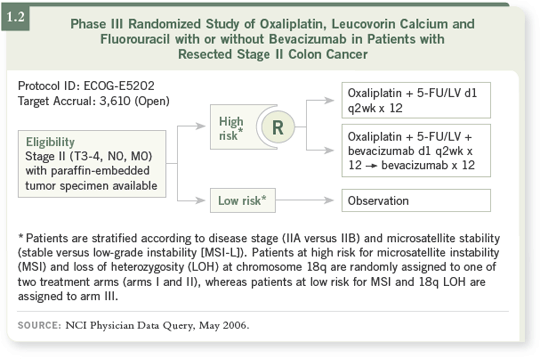
An important clinical trial is being performed in ECOG and through the
cooperative groups that’s evaluating risk assessment of Stage II patients based
on 18q deletion and microsatellite instability (MSI; [1.2]).
If patients have favorable characteristics for microsatellite instability —
basically, MSI high and no 18q deletion — they are assigned to observation
only.
If patients have one or two bad characteristics from this molecular analysis,
they will be randomly assigned to either six months of FOLFOX or six
months of FOLFOX and bevacizumab.
 DR LOVE: How do you approach the treatment of Stage II disease off
protocol?
DR LOVE: How do you approach the treatment of Stage II disease off
protocol?
 DR ROTHENBERG: I would probably look at these characteristics, although
I have been in situations in which test results have come back conflicting
— one criterion suggesting a good-prognosis tumor and another suggesting a
poor-prognosis tumor. So it has ended up being rather puzzling and confusing
to me.
DR ROTHENBERG: I would probably look at these characteristics, although
I have been in situations in which test results have come back conflicting
— one criterion suggesting a good-prognosis tumor and another suggesting a
poor-prognosis tumor. So it has ended up being rather puzzling and confusing
to me.
I hope the results of the clinical trial that I just described will help. Often, the
situation is resolved by talking to the patients and finding out whether they are
most concerned about side effects from treatment and inconvenience, whether
they feel comfortable in a watch-and-wait mode or whether they want to go
to great lengths to try and eradicate every last cancer cell that might be lying
dormant and are willing to withstand four to six months of adjuvant treatment
along with the potential risks and side effects.
I try to use what patients tell me to determine the recommendation for
whether they receive treatment or not. I can’t say that I follow a “one-size-fits-all” approach when it comes to Stage II colon cancer.
Track 7
 DR LOVE:
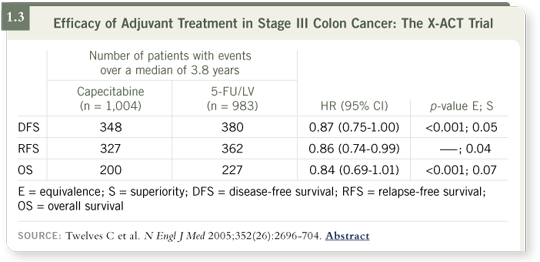
DR LOVE: Can you discuss the X-ACT trial (Twelves 2005)?
 DR ROTHENBERG: This study compared single-agent capecitabine to the
Mayo Clinic regimen of 5-FU/leucovorin for patients with locally advanced
colon cancer. It was conducted as a noninferiority trial to find out whether
5-FU and leucovorin could be replaced by an oral agent. The bottom line was
that it could.
DR ROTHENBERG: This study compared single-agent capecitabine to the
Mayo Clinic regimen of 5-FU/leucovorin for patients with locally advanced
colon cancer. It was conducted as a noninferiority trial to find out whether
5-FU and leucovorin could be replaced by an oral agent. The bottom line was
that it could.
In some measures of activity, capecitabine was trending toward being a little
bit better than 5-FU/leucovorin (1.3).
I believe this is a very important trial — it’s been published in The New
England Journal of Medicine (Twelves 2005) — and it showed that we have an
option for individuals who may not want to come into a clinic on a weekly
basis because of their work or travel schedules and are willing and able to
comply with an oral dosing regimen of capecitabine.
The side effects associated with capecitabine are different from those of the
bolus 5-FU. You have a little bit less mucositis and myelosuppression, but you
have a little bit more in the way of hand-foot syndrome.
It is important to note that both regimens can result in diarrhea. So patients
must understand which side effects they’re most likely to encounter, whom to
contact and what to do about those side effects.
Track 8
 DR LOVE:
DR LOVE: How do you approach the dosing of capecitabine in the
adjuvant setting?
 DR ROTHENBERG: That’s a tough question because I’m always concerned
about citing statistics from a trial and then not giving the same doses that were
given in the trial. I would probably not start at 2,500 mg/m2* but at 2,000
mg/m2 and then monitor the patient very closely. If the patient tolerated the
drug well, I would escalate the dose to 2,500 mg/m2 by the second cycle. If he
or she were having some side effects of concern but they were manageable, I’d
stay at 2,000 mg/m2.
DR ROTHENBERG: That’s a tough question because I’m always concerned
about citing statistics from a trial and then not giving the same doses that were
given in the trial. I would probably not start at 2,500 mg/m2* but at 2,000
mg/m2 and then monitor the patient very closely. If the patient tolerated the
drug well, I would escalate the dose to 2,500 mg/m2 by the second cycle. If he
or she were having some side effects of concern but they were manageable, I’d
stay at 2,000 mg/m2.
The key is to dose at the optimum or maximum-tolerated dose for each
patient. If the patient is experiencing tolerable Grade II toxicities, he or she is
receiving enough of the drug.
* Two divided doses for 14 of 21 days
Select publications

