
| Tracks 1-14 |
| Track 1 |
Introduction |
| Track 2 |
Evaluating resectability of hepatic
metastases |
| Track 3 |
Utility of radiofrequency ablation
for hepatic metastases |
| Track 4 |
NSABP-C-09: CAPOX with
hepatic arterial infusion of FUDR
versus CAPOX for patients with
resected or ablated hepatic
metastases |
| Track 5 |
Quality control among surgical
oncologists treating colon cancer |
| Track 6 |
Combined-modality preoperative
chemoradiation therapy for rectal
cancer |
| Track 7 |
Clinical evaluation and staging of
rectal cancer |
| Track 8 |
Evaluation of patient suitability for
abdominal perineal resection |
|
| Track 9 |
NSABP-R-04: Preoperative
radiation therapy and
capecitabine with or without
oxaliplatin or continuous infusion
5-FU with or without oxaliplatin in
rectal cancer |
| Track 10 |
Surgical considerations in open
versus laparoscopic colectomy |
| Track 11 |
Factors related to the adequacy of
nodal sampling |
| Track 12 |
Multimodality management of
early colon cancer |
| Track 13 |
NSABP-C-10 trial for patients
with synchronous primary and
metastatic disease |
| Track 14 |
Treatment of patients presenting
with both primary and resectable
metastatic disease |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: For patients with liver-only metastases, how do you determine
whether the disease is resectable, possibly resectable in the future or never
resectable?
 DR PETRELLI: When patients come to me for a possible liver resection, I’m
looking for a reason not to operate. You need to ascertain whether the disease
in their liver looks like resectable disease, which depends on the size and
number of lesions. But the most important question is, does the patient have
any evidence of extrahepatic disease?
DR PETRELLI: When patients come to me for a possible liver resection, I’m
looking for a reason not to operate. You need to ascertain whether the disease
in their liver looks like resectable disease, which depends on the size and
number of lesions. But the most important question is, does the patient have
any evidence of extrahepatic disease?
In my mind and I think most of my colleagues’ minds — although a shift is
occurring — extrahepatic disease is a contraindication to resection outside any
type of protocol. I know an undercurrent exists, because of the new agents, to
take patients with extrahepatic disease and resect their primary tumor along
with their liver disease, but I believe that should be done as part of a clinical
trial.
 DR LOVE: Can you talk about those patients with disease you consider
unresectable at the moment but that has the potential for resectability?
DR LOVE: Can you talk about those patients with disease you consider
unresectable at the moment but that has the potential for resectability?
 DR PETRELLI: Those patients need a true multidisciplinary approach to their
disease. They must have input from the surgeon and the medical oncologist.
Those individuals will likely be treated up front with FOLFOX and
bevacizumab. They have to be followed closely because a window of opportunity
will arise when those cases are converted from unresectable to resectable.
Constant communication is necessary between the medical and surgical
oncologists. After the first two cycles, the patient should be reevaluated to
determine if the disease has become resectable.
DR PETRELLI: Those patients need a true multidisciplinary approach to their
disease. They must have input from the surgeon and the medical oncologist.
Those individuals will likely be treated up front with FOLFOX and
bevacizumab. They have to be followed closely because a window of opportunity
will arise when those cases are converted from unresectable to resectable.
Constant communication is necessary between the medical and surgical
oncologists. After the first two cycles, the patient should be reevaluated to
determine if the disease has become resectable.
Track 9
 DR LOVE:
DR LOVE: NSABP-R-04 originally compared neoadjuvant radiation
therapy in combination with continuous infusion 5-FU or capecitabine,
and a second question concerning the role of oxaliplatin has been added.
What are your thoughts about this trial?
 DR PETRELLI: I believe NSABP-R-04 is a very important study. It goes back
to the issue of a prospective, randomized, multicenter trial. I’ve been in this
business long enough to know that data from a single-institution trial have to
be reproduced.
DR PETRELLI: I believe NSABP-R-04 is a very important study. It goes back
to the issue of a prospective, randomized, multicenter trial. I’ve been in this
business long enough to know that data from a single-institution trial have to
be reproduced.
I believe the addition of oxaliplatin is important, and it didn’t increase the
target accrual because we’re talking about a noninferiority trial for the
capecitabine versus continuous infusion 5-FU comparison. The accrual has
been going very well for NSABP-R-04, even before oxaliplatin was added.
 DR LOVE: If capecitabine is equivalent to infusional 5-FU, it will be a big
boost to the patient in terms of avoiding the pump, et cetera.
DR LOVE: If capecitabine is equivalent to infusional 5-FU, it will be a big
boost to the patient in terms of avoiding the pump, et cetera.
 DR PETRELLI: Sure. It’s a good quality-of-life question.
DR PETRELLI: Sure. It’s a good quality-of-life question.
Track 13
 DR LOVE:
DR LOVE: Can you talk about the NSABP-C-10 trial?
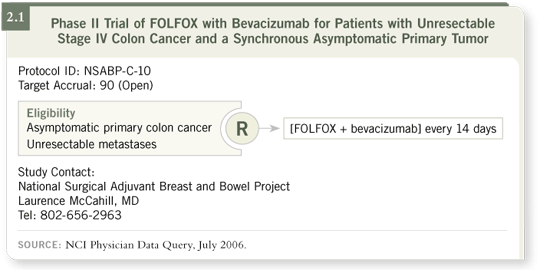
 DR PETRELLI: NSABP-C-10 is a Phase II trial for patients who present with
endoscopically detected, asymptomatic primary colon cancer and unresectable
distant metastases (2.1). This trial evaluates the hypothesis that by treating
those patients with chemotherapy and bevacizumab, you don’t have to remove the primary tumor. I believe that’s a very provocative and practical question
because about 60 percent of these patients, according to SEER data, are having
their primary tumors removed (Cook 2005).
DR PETRELLI: NSABP-C-10 is a Phase II trial for patients who present with
endoscopically detected, asymptomatic primary colon cancer and unresectable
distant metastases (2.1). This trial evaluates the hypothesis that by treating
those patients with chemotherapy and bevacizumab, you don’t have to remove the primary tumor. I believe that’s a very provocative and practical question
because about 60 percent of these patients, according to SEER data, are having
their primary tumors removed (Cook 2005).
The primary aim of the study is to observe the incidence of obstruction,
perforation, fistula formation and hemorrhage that requires surgery. The
secondary aim is to assess those potential complications with which the patient
may not require surgery but needs hospitalization.
 DR LOVE: If you see that the patients respond well with very low rates of local
complications, what do you think will be the next step?
DR LOVE: If you see that the patients respond well with very low rates of local
complications, what do you think will be the next step?
 DR PETRELLI: If we see what you describe, I don’t believe we’ll have to do a
Phase III trial. I believe we could probably state that if a patient presents with
an asymptomatic primary tumor in the colon, not the rectum, you don’t have
to worry about removing the primary tumor.
DR PETRELLI: If we see what you describe, I don’t believe we’ll have to do a
Phase III trial. I believe we could probably state that if a patient presents with
an asymptomatic primary tumor in the colon, not the rectum, you don’t have
to worry about removing the primary tumor.
In this Phase II trial, a small number of patients will probably receive
chemotherapy and their liver disease will become resectable. I wouldn’t be
surprised if we saw some of those patients undergoing a resection of both their
primary and distant disease.
Select publications

