
| Tracks 1-15 |
| Track 1 |
Introduction |
| Track 2 |
Adjuvant therapy for patients with
Stage II colon cancer |
| Track 3 |
Relative contraindications to the
use of adjuvant oxaliplatin |
| Track 4 |
Use of adjuvant fluoropyrimidine
monotherapy |
| Track 5 |
Use of adjuvant irinotecan-containing
regimens |
| Track 6 |
Increasing resectability of hepatic
metastases |
| Track 7 |
Incorporation of biologic therapies
into neoadjuvant chemotherapy
regimens |
| Track 8 |
Background and rationale
for the NSABP-C-09 study of
capecitabine, oxaliplatin and
FUDR |
|
| Track 9 |
Achieving durable control or cure in patients with hepatic metastases |
| Track 10 |
Rationale for incorporation of
capecitabine and oxaliplatin into
NSABP-C-09 |
| Track 11 |
Side effects and tolerability of
CAPOX and intrahepatic FUDR |
| Track 12 |
Radiofrequency ablation in the
treatment of hepatic metastases |
| Track 13 |
Advantages of active patient
surveillance after adjuvant
therapy |
| Track 14 |
Treatment of isolated extrahepatic
metastases |
| Track 15 |
Future directions in clinical
research: Targeted and individualized
therapy |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: How do you approach the treatment of patients with Stage II
colon cancer?
 DR ALBERTS: Generally, I tend to follow the Mayo Clinic approach of
considering ploidy status, tumor size, angiolymphatic invasion and adequacy of
node assessment as risk factors in deciding whether or not to treat a patient. If
a patient has a diploid tumor and an adequate lymph node assessment, I often
recommend observation.
DR ALBERTS: Generally, I tend to follow the Mayo Clinic approach of
considering ploidy status, tumor size, angiolymphatic invasion and adequacy of
node assessment as risk factors in deciding whether or not to treat a patient. If
a patient has a diploid tumor and an adequate lymph node assessment, I often
recommend observation.
 DR LOVE: What is your specific choice of adjuvant therapy for patients with
Stage II disease?
DR LOVE: What is your specific choice of adjuvant therapy for patients with
Stage II disease?
 DR ALBERTS: If I were going to treat somebody, given the lack of information,
I would certainly treat with FOLFOX. If you’re going to make that
commitment, it’s hard to justify treating somebody with something that’s
potentially an inferior regimen.
DR ALBERTS: If I were going to treat somebody, given the lack of information,
I would certainly treat with FOLFOX. If you’re going to make that
commitment, it’s hard to justify treating somebody with something that’s
potentially an inferior regimen.
 DR LOVE: How do you approach the management of patients who have a
suboptimal number of nodes identified?
DR LOVE: How do you approach the management of patients who have a
suboptimal number of nodes identified?
 DR ALBERTS: For patients who may come in with 10 or fewer involved
lymph nodes, I always explain that, given the few lymph nodes that were
removed or assessed, it may be a little harder to determine whether they
indeed have Stage II or, potentially, Stage III disease. In that setting, I may be
more likely to advocate the use of adjuvant therapy.
DR ALBERTS: For patients who may come in with 10 or fewer involved
lymph nodes, I always explain that, given the few lymph nodes that were
removed or assessed, it may be a little harder to determine whether they
indeed have Stage II or, potentially, Stage III disease. In that setting, I may be
more likely to advocate the use of adjuvant therapy.
Track 4
 DR LOVE:
DR LOVE: In general, how do you decide between 5-FU and capecitabine
if you’re going to use fluoropyrimidine monotherapy?
 DR ALBERTS: Part of the decision is related to convenience and the other is
related to monitoring of therapy. At least in my experience, capecitabine has
manageable side effects if the patient understands the need to hold the therapy
if he or she develops hand-foot syndrome or other side effects.
DR ALBERTS: Part of the decision is related to convenience and the other is
related to monitoring of therapy. At least in my experience, capecitabine has
manageable side effects if the patient understands the need to hold the therapy
if he or she develops hand-foot syndrome or other side effects.
Certainly, some patients do go on to develop significant side effects despite
stopping the therapy, but capecitabine is convenient, and its efficacy is equivalent
to 5-FU/leucovorin. Thus, the ability to take pills home and not have
to come into the office for either the Mayo Clinic regimen or weekly 5-FU/leucovorin makes capecitabine a lot easier for the patients.
Track 10
 DR LOVE:
DR LOVE: What is the rationale for using capecitabine with oxaliplatin in
your pilot study of hepatic-only disease and in the subsequent NSABP-C-
09 study?
 DR ALBERTS: Several thoughts went into making this decision. One was the
observation that through the three-step process of metabolizing capecitabine,
you achieve higher hepatic levels of the active metabolite in tumor cells versus
nontumor cells, and the hope was that this would enhance the response rate.
DR ALBERTS: Several thoughts went into making this decision. One was the
observation that through the three-step process of metabolizing capecitabine,
you achieve higher hepatic levels of the active metabolite in tumor cells versus
nontumor cells, and the hope was that this would enhance the response rate.
The other consideration was convenience for the patients. Compared to bolus
5-FU, capecitabine seemed to provide a more convenient method of administration,
and it also simulated infusional 5-FU because it was being administered
over two weeks.
At this point, it’s still unclear whether capecitabine/oxaliplatin is equivalent
to infusional 5-FU/oxaliplatin. A number of randomized Phase II trials,
including the TREE-1 trial, suggest they’re comparable (Hochster 2005,
2006).
Track 11
 DR LOVE:
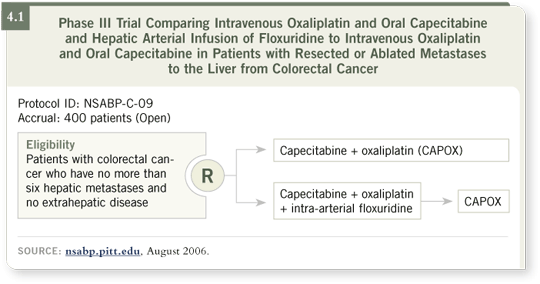
DR LOVE: In your Phase II trial, what did you see in terms of side effects
and toxicity when you combined CAPOX and intrahepatic FUDR for
patients with surgically resected liver metastases (4.1)?
 DR ALBERTS: Primarily, as patients completed the FUDR infusion, had a
one-week rest and then went on to receive capecitabine/oxaliplatin, a little
bump in liver enzymes occurred (Alberts 2006). The thought was that because
of the metabolism of the drug in the liver, some interaction with FUDR was
causing mild but subclinical irritation of the liver. Adding the capecitabine
enhanced that effect, and that was one reason we reduced the dose from 1,000
mg/m2 twice to 850 mg/m2 twice a day.
DR ALBERTS: Primarily, as patients completed the FUDR infusion, had a
one-week rest and then went on to receive capecitabine/oxaliplatin, a little
bump in liver enzymes occurred (Alberts 2006). The thought was that because
of the metabolism of the drug in the liver, some interaction with FUDR was
causing mild but subclinical irritation of the liver. Adding the capecitabine
enhanced that effect, and that was one reason we reduced the dose from 1,000
mg/m2 twice to 850 mg/m2 twice a day.
Beyond that, we didn’t see an increase in events such as neutropenia, with
the belief that very little of the FUDR gets out into the systemic circulation.
Because we were administering a two-week infusion of FUDR followed by a
one-week break and then the capecitabine/oxaliplatin for two weeks followed
by a one-week break, a long lag existed between the systemic therapies.
Beyond some mild increase in liver enzymes, we didn’t see any apparent
synergistic toxicities between the two treatments.
Select publications

