
| Tracks 1-11 |
| Track 1 |
Introduction |
| Track 2 |
MD Anderson study of FOLFIRI
with bevacizumab |
| Track 3 |
Effects of bevacizumab on tumor
blood flow |
| Track 4 |
Clinical implications of the MD
Anderson FOLFIRI/bevacizumab
study |
| Track 5 |
Selection of first-line therapy for
chemotherapy-naïve patients |
| Track 6 |
Treatment after discontinuation
of oxaliplatin due to neurotoxicity
followed by progression on
bevacizumab |
|
| Track 7 |
MD Anderson retrospective
analysis of patients treated with
bevacizumab |
| Track 8 |
Effects of bevacizumab on wound
healing and bowel perforation |
| Track 9 |
AVANT adjuvant study comparing
FOLFOX to FOLFOX or CAPOX
with bevacizumab |
| Track 10 |
Ongoing adjuvant trials in colon
cancer: NCCTG-N0147 and
NSABP-C-08 |
| Track 11 |
MD Anderson preoperative study
of capecitabine/bevacizumab with
radiation therapy |
|
|
Select Excerpts from the Interview
Track 6
 DR LOVE:
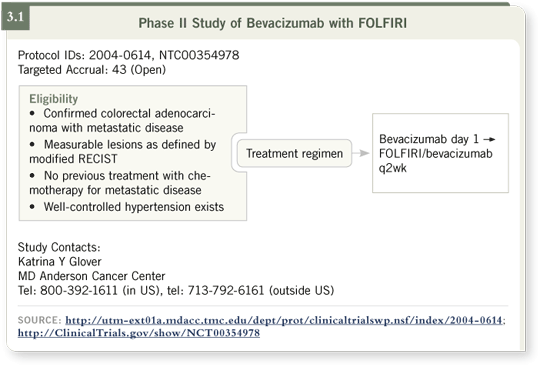
DR LOVE: Can you discuss your FOLFIRI/bevacizumab study (3.1)?
 DR HOFF: We now know that IFL with bevacizumab is better than IFL alone
(Hurwitz 2004) and that FOLFIRI is less toxic and more efficacious than IFL,
but we do not have any trial data on the efficacy of adding bevacizumab to
FOLFIRI.
DR HOFF: We now know that IFL with bevacizumab is better than IFL alone
(Hurwitz 2004) and that FOLFIRI is less toxic and more efficacious than IFL,
but we do not have any trial data on the efficacy of adding bevacizumab to
FOLFIRI.
So we decided to conduct a Phase II trial evaluating the combination of
FOLFIRI and bevacizumab (Hoff 2006). We are also collecting blood from
the study participants for proteomic analysis and are doing DC MRIs to look
at blood flow and changes in blood flow with bevacizumab alone or bevacizumab
with FOLFIRI.
The trial has an interesting design. To conduct these correlative studies, patients
receive only bevacizumab in the first cycle of treatment. We call that day
minus 14 when they receive bevacizumab alone. After two weeks, the patients begin treatment with FOLFIRI and bevacizumab. This allows us to have information
from blood and imaging of the use of bevacizumab alone and in combination
with FOLFIRI. It’s something that I believe will allow us to learn a lot
about the interaction of the drugs and what happens within the tumor when
the drugs are taken. Among the first 20 patients we have evaluated so far, we
have reached a response rate of 70 percent. For us, this is very good.
Although patients have diarrhea because of the use of irinotecan, it has been
relatively straightforward to control. We have seen the expected incidence
of neutropenia, but the percentages of Grade III and IV toxicities have been
in line with those previously reported with FOLFIRI. It seems that adding
bevacizumab has not increased toxicity tremendously but has increased the
efficacy of the regimen.
The primary endpoint for the trial is progression-free survival, although we
are following the response rate. This trial has been open for more than one
year now and we still do not yet have median progression-free survival data,
but we hope to achieve a progression-free survival that will be significantly
longer than what we used to see with FOLFIRI alone.
Track 4
 DR LOVE:
DR LOVE: What are the practical clinical implications of this study?
 DR HOFF: If our results are maintained with this high response rate and
prolonged progression-free survival with acceptable toxicity, it will be an
indication that the use of FOLFIRI with bevacizumab is a very good option in
front-line treatment for metastatic colorectal cancer.
DR HOFF: If our results are maintained with this high response rate and
prolonged progression-free survival with acceptable toxicity, it will be an
indication that the use of FOLFIRI with bevacizumab is a very good option in
front-line treatment for metastatic colorectal cancer.
One important point in favor of FOLFIRI with bevacizumab is that we do
not encounter a cumulative toxicity that will necessitate patients changing
therapy after a set number of cycles. Of course, we do see fatigue and neutropenia
with FOLFIRI, but once the patients reach a dose that their organs are
used to, I believe they can tolerate this regimen for quite some time.
An important point is that we have seen late responses with this combination.
We are used to seeing a maximal response around two months after we
start chemotherapy. In our trial, many of the responses that reached RECIST
were seen later. Our median time to response in this trial is 18 weeks. This
is very encouraging because I believe it is due to the impact of bevacizumab,
and perhaps long-term benefits will continue to be seen in patients who are on
treatment.
Tracks 5-6
 DR LOVE:
DR LOVE: What tends to be your first-line therapy for metastatic disease
in a patient who is chemotherapy naïve?
 DR HOFF: I’m increasingly tailoring therapy for individual patients. I discuss
with my patients that the two regimens I most commonly use right now are
FOLFIRI or FOLFOX with bevacizumab, provided patients have no contraindications
to the use of the bevacizumab or any of the other agents.
DR HOFF: I’m increasingly tailoring therapy for individual patients. I discuss
with my patients that the two regimens I most commonly use right now are
FOLFIRI or FOLFOX with bevacizumab, provided patients have no contraindications
to the use of the bevacizumab or any of the other agents.
If I have a patient who has a disease that may become operable within a
relatively short period of time, I often use FOLFOX and bevacizumab. With
patients for whom I expect the treatment to be necessary for longer periods of
time, I often use FOLFIRI and bevacizumab. I believe the use of FOLFOX or
FOLFIRI is a decision that must be made based on physician comfort with the
regimens because the efficacy between the two combinations is very similar.
 DR LOVE: What about CAPIRI or CAPOX?
DR LOVE: What about CAPIRI or CAPOX?
 DR HOFF: CAPIRI and CAPOX are very good options. We have less experience
with the use of CAPIRI or CAPOX with bevacizumab here in the
United States. I believe the way bevacizumab was approved in the United
States directed us more to using combinations with infusional 5-FU than with
capecitabine, which is an excellent agent.
DR HOFF: CAPIRI and CAPOX are very good options. We have less experience
with the use of CAPIRI or CAPOX with bevacizumab here in the
United States. I believe the way bevacizumab was approved in the United
States directed us more to using combinations with infusional 5-FU than with
capecitabine, which is an excellent agent.
Recently, we have had patients who were treated with CAPOX and bevacizumab.
Although it’s difficult to compare efficacy between these two
regimens because we only have personal experience and no randomized trial
experience, our results have been similar to what we would expect to see with
FOLFOX and bevacizumab. I feel comfortable with these agents in combination
with bevacizumab, particularly CAPOX, with which we have more
experience from other centers.
 DR LOVE: How do you treat a patient who has responded well to FOLFOX
and bevacizumab, is eventually taken off oxaliplatin because of neurotoxicity,
continues with 5-FU/bevacizumab, and then experiences disease progression
at 18 months?
DR LOVE: How do you treat a patient who has responded well to FOLFOX
and bevacizumab, is eventually taken off oxaliplatin because of neurotoxicity,
continues with 5-FU/bevacizumab, and then experiences disease progression
at 18 months?
 DR HOFF: This is an important question because FOLFOX or CAPOX with
bevacizumab are probably the most commonly used regimens in the United
States. We know that patients treated with these regimens do not progress
within the first few months and that the majority will still derive benefit from
the treatment when they develop too much neuropathy to be able to continue
with oxaliplatin. We often stop oxaliplatin and continue with a fluoropyrimidine
and bevacizumab.
DR HOFF: This is an important question because FOLFOX or CAPOX with
bevacizumab are probably the most commonly used regimens in the United
States. We know that patients treated with these regimens do not progress
within the first few months and that the majority will still derive benefit from
the treatment when they develop too much neuropathy to be able to continue
with oxaliplatin. We often stop oxaliplatin and continue with a fluoropyrimidine
and bevacizumab.
Those patients eventually will progress, although some patients derive great
benefit for long periods of time just from the fluoropyrimidine and bevacizumab.
We have two ways of approaching these patients at this point. Those
patients who rapidly recover from the peripheral neuropathy from oxaliplatin
could be reexposed to a combination with oxaliplatin, more or less following
the OPTIMOX design that has been recently published by the group from Dr
de Gramont (Tournigand 2006).
 DR LOVE: Continuing the bevacizumab?
DR LOVE: Continuing the bevacizumab?
 DR HOFF: If you’re going to reintroduce the oxaliplatin, that’s one option,
although we do not know about the benefit of continuing bevacizumab,
particularly in this situation. Since patients were previously responding to a
combination with oxaliplatin and bevacizumab, however, it would be reasonable
to continue in this particular situation.
DR HOFF: If you’re going to reintroduce the oxaliplatin, that’s one option,
although we do not know about the benefit of continuing bevacizumab,
particularly in this situation. Since patients were previously responding to a
combination with oxaliplatin and bevacizumab, however, it would be reasonable
to continue in this particular situation.
However, for those patients who still have significant peripheral neuropathy
and for whom reintroduction of oxaliplatin would not be reasonable, I tend
to switch completely to an irinotecan-based regimen, and usually I do not
continue bevacizumab in that situation.
Tracks 9-10
 DR LOVE:
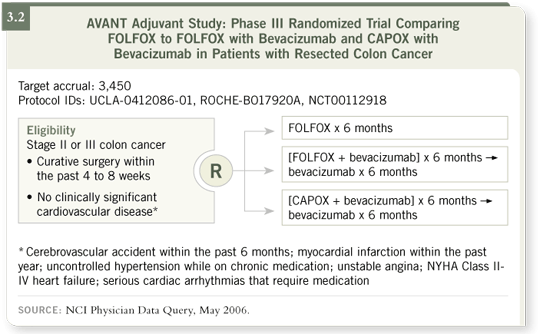
DR LOVE: Can you comment on the AVANT trial?
 DR HOFF: The AVANT trial is one of the most important ongoing trials right
now. It’s for patients who have high-risk Stage II or Stage III colon cancer
(3.2). After surgery, patients are randomly assigned to receive FOLFOX4 for
six months, which is considered the standard of care for those patients right
now. This trial has two experimental arms. In the first arm, patients receive
FOLFOX4 and bevacizumab for six months and then six more months of
bevacizumab alone. In the second arm, patients receive CAPOX with bevacizumab
for six months followed by another six months of bevacizumab.
DR HOFF: The AVANT trial is one of the most important ongoing trials right
now. It’s for patients who have high-risk Stage II or Stage III colon cancer
(3.2). After surgery, patients are randomly assigned to receive FOLFOX4 for
six months, which is considered the standard of care for those patients right
now. This trial has two experimental arms. In the first arm, patients receive
FOLFOX4 and bevacizumab for six months and then six more months of
bevacizumab alone. In the second arm, patients receive CAPOX with bevacizumab
for six months followed by another six months of bevacizumab.
The objective is to determine if the addition of bevacizumab adds to the
benefit of an oxaliplatin and fluoropyrimidine regimen in this setting and
whether we can use CAPOX instead of FOLFOX. CAPOX offers some
significant advantages in convenience, but I remind you that if we use
CAPOX, the cumulative dose of oxaliplatin may be slightly less than that
from administering 12 cycles of FOLFOX because you only use eight cycles
with CAPOX.
 DR LOVE: Why was the decision made to administer eight cycles of CAPOX?
DR LOVE: Why was the decision made to administer eight cycles of CAPOX?
 DR HOFF: That’s six months of therapy, and the idea was to continue to use
the same regimens with which we have experience. The X-ACT trial was
eight cycles of capecitabine versus the Roswell Park regimen. The CAPOX
trial in the adjuvant setting is eight cycles, which ended up being the six
months that we have come to accept as necessary for those patients. One could
argue that perhaps six months is too much. We do have the British trial in
which three months of infusional 5-FU was similar to six months of 5-FU/leucovorin. But I think that right now, worldwide, the feeling is that until we
have further data, six months should be considered our standard.
DR HOFF: That’s six months of therapy, and the idea was to continue to use
the same regimens with which we have experience. The X-ACT trial was
eight cycles of capecitabine versus the Roswell Park regimen. The CAPOX
trial in the adjuvant setting is eight cycles, which ended up being the six
months that we have come to accept as necessary for those patients. One could
argue that perhaps six months is too much. We do have the British trial in
which three months of infusional 5-FU was similar to six months of 5-FU/leucovorin. But I think that right now, worldwide, the feeling is that until we
have further data, six months should be considered our standard.
Select publications

