You are here: Home: CCU 1 | 2006: Herbert Hurwitz, MD

Tracks 1-20 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Bevacizumab-associated
hypertension |
| Track 3 |
Clinical evaluation of risk for
bevacizumab-associated
arteriovascular events |
| Track 4 |
Potential cardiovascular effects
of bevacizumab |
| Track 5 |
Potential factors contributing to
bowel perforations in studies
of bevacizumab |
| Track 6 |
Bevacizumab-associated
bleeding complications |
| Track 7 |
Management of
bowel-related complications |
| Track 8 |
Timing of bevacizumab administration
and surgery |
| Track 9 |
Effect of bevacizumab on
hepatic regeneration |
| Track 10 |
Role of VEGF inhibition in the
adjuvant setting |
|
| Track 11 |
Potential mechanisms of action
of bevacizumab |
| Track 12 |
Clinical use of
adjuvant bevacizumab |
| Track 13 |
Administration of bevacizumab
beyond chemotherapy in the
adjuvant setting |
| Track 14 |
Clinical activity of
single-agent bevacizumab |
| Track 15 |
Continuation of bevacizumab
after disease progression |
| Track 16 |
Determining the optimal dose
of bevacizumab |
| Track 17 |
Evaluation of cetuximab and
bevacizumab in combination |
| Track 18 |
Management of bevacizumab-related
side effects |
| Track 19 |
Pharmacoeconomic evaluation
of novel therapies |
| Track 20 |
Combining capecitabine
with bevacizumab |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: Can you summarize the current available database on the
safety of bevacizumab? DR LOVE: Can you summarize the current available database on the
safety of bevacizumab? |
 DR HURWITZ: So far, the main side effect has been hypertension that, in
general, is modest and easily manageable. It occurs in about a quarter of
all patients, and perhaps one, two or three in 10 patients will need to start
receiving blood pressure medication or will need an adjustment to a component
of their blood pressure regimen. In general, all blood pressure medications used to date have been successful. The key issue is close monitoring of
the patient. DR HURWITZ: So far, the main side effect has been hypertension that, in
general, is modest and easily manageable. It occurs in about a quarter of
all patients, and perhaps one, two or three in 10 patients will need to start
receiving blood pressure medication or will need an adjustment to a component
of their blood pressure regimen. In general, all blood pressure medications used to date have been successful. The key issue is close monitoring of
the patient.
As oncologists, we used to be able to forget about many general internal
medicine issues because cancer mortality was the most important issue for our
patients. Now that patients are able to stay on treatments for a year and longer
in some cases, management of side effects will be important.
In particular, blood pressure should probably be measured at every clinic visit,
and if a clear trend toward increased blood pressure appears, medication should
be instituted. Best clinical judgment should be used in the selection and titration
of any agent.
 DR LOVE: Is anything new in terms of what is known about the mechanism
of developing hypertension? DR LOVE: Is anything new in terms of what is known about the mechanism
of developing hypertension?
 DR HURWITZ: The mechanism is not yet well understood. Preclinical models
and some experience in the cardiovascular literature suggest that nitric oxide-mediated
mechanisms are likely involved (Shen 1999). DR HURWITZ: The mechanism is not yet well understood. Preclinical models
and some experience in the cardiovascular literature suggest that nitric oxide-mediated
mechanisms are likely involved (Shen 1999).
Nitric oxide regulates many processes, including those related to vascular tone,
renal hemodynamics and alterations of the renin-angiotensin system. In short,
we don’t yet know the mechanism of hypertension in these patients. More
than likely, some component of all of these processes is involved.
At this time, we don’t have enough mechanistic information to drive the
selection of one antihypertensive medication over another. Currently, I suggest
making this decision based on other reasons one drug may be preferred versus
another — the way we do with all patients.
 Tracks 4 and 6 Tracks 4 and 6
 DR LOVE: Can you discuss the issues related to bowel perforation and
bevacizumab? What have we learned over the last year or two? DR LOVE: Can you discuss the issues related to bowel perforation and
bevacizumab? What have we learned over the last year or two? |
 DR HURWITZ: The issues related to bowel perforation have many of the same
general themes as the arteriovascular event risks. In general, these risks are low.
Several studies of bevacizumab — the large IFL study (Hurwitz 2004), the
Phase II 5-FU/leucovorin study (Kabbinavar 2005) and the ECOG second-line
FOLFOX with or without bevacizumab study (Giantonio 2005) — have
reported a one to two percent risk of GI perforation. DR HURWITZ: The issues related to bowel perforation have many of the same
general themes as the arteriovascular event risks. In general, these risks are low.
Several studies of bevacizumab — the large IFL study (Hurwitz 2004), the
Phase II 5-FU/leucovorin study (Kabbinavar 2005) and the ECOG second-line
FOLFOX with or without bevacizumab study (Giantonio 2005) — have
reported a one to two percent risk of GI perforation.
A similar risk is apparent in the historic literature — anywhere between two
to five percent of some type of bowel perforation, abscess or fistula formation
with the use of chemotherapy alone.
The reasons for those findings are not well understood. These problems
have not been observed as commonly in other tumor settings, aside from the
ovarian cancer population. This may relate to the fact that GI-toxic regimens
are used for colon cancer — more diarrhea and bowel inflammation and inflammatory wound-healing responses, in general, may predispose these
patients to perforation.
 DR LOVE: As bevacizumab data accumulate, have we learned anything more
about the clinical presentation of these bowel perforations? DR LOVE: As bevacizumab data accumulate, have we learned anything more
about the clinical presentation of these bowel perforations?
 DR HURWITZ: For the most part, the presentation of bowel perforation is
what you would expect — essentially like an acute abdomen. The definition
of bowel perforation, though, has often been very liberal to allow us to detect
any event that might be even partially implicated. DR HURWITZ: For the most part, the presentation of bowel perforation is
what you would expect — essentially like an acute abdomen. The definition
of bowel perforation, though, has often been very liberal to allow us to detect
any event that might be even partially implicated.
As cancer doctors, we’re quite used to dealing with the complications of
regimens that affect bowel integrity. IFL, FOLFOX, and 5-FU alone all cause
diarrhea, and if that gets out of hand, it can lead to an occasional serious or
even life-threatening complication.
Patients who have bowel trouble and start to experience serious symptoms
should be evaluated urgently, and standard management should be pursued.
 Tracks 8-9 Tracks 8-9
 DR LOVE: What do we know about patients who require emergent
surgery while they’re receiving bevacizumab? DR LOVE: What do we know about patients who require emergent
surgery while they’re receiving bevacizumab? |
 DR HURWITZ: The issue of surgery and bevacizumab needs to be broken
down into two separate categories. One category includes patients who have
major surgery and then receive bevacizumab. DR HURWITZ: The issue of surgery and bevacizumab needs to be broken
down into two separate categories. One category includes patients who have
major surgery and then receive bevacizumab.
Among patients who’ve waited at least a month prior to starting bevacizumab
and who have healed sufficiently to be cleared by their surgeons, no increased
risk of wound-healing complications is apparent. The converse setting, where
patients are on bevacizumab and then go to surgery, is a bit more complicated.
The best data come from the IFL study (Hurwitz 2004). Most of the patients
going to surgery did so for an urgent or emergent indication — usually major
abdominal procedures.
Wound-healing complications ranged from minor to major wound dehiscence
or major bruising at the site, and the wound-healing complication risk ranged
from about four to 14 percent.
Most complications were manageable — rarely lethal. I would interpret that
range in risk to mean that the rate does increase for emergent or urgent
indications, such as drainage of an abscess or some other acute issue. That risk
is acceptable, and the risk of wound-healing complications should not deter
good surgical or interventional management when needed.
For highly elective surgery, one should respect the half-life of the drug, which
is about 21 days. One should wait two to three half-lives of the drug — about
40 to 60 days — before elective surgery. This becomes a bit of a risk-benefit
ratio, as we have not truly identified the best interval for timing elective metastatic resection. I think the window for metastatic resections is probably
about six weeks following cessation of treatment with bevacizumab.
 DR LOVE: With respect to hepatic resection or ablation, what do we know
about the effect of bevacizumab on hepatic regeneration? DR LOVE: With respect to hepatic resection or ablation, what do we know
about the effect of bevacizumab on hepatic regeneration?
 DR HURWITZ: We don’t know a lot about the effect of bevacizumab per se
on hepatic regeneration. From clinical and preclinical information, we know
that hepatic regeneration is angiogenesis-dependent. DR HURWITZ: We don’t know a lot about the effect of bevacizumab per se
on hepatic regeneration. From clinical and preclinical information, we know
that hepatic regeneration is angiogenesis-dependent.
We also know that the majority of hepatic regeneration occurs within a few
weeks to a month or so after surgery. Some patients clearly take a little bit
longer to regain both liver volume and function after major resection, particularly
after a right hepatectomy.
In general, the patient should be well healed, should have normal liver
function test results, and should be cleared by his or her surgeon before
starting bevacizumab. I think a patient who’s completely healed and who has
normal liver function test results is a suitable candidate.
Allow two months, perhaps three months for some patients, to recuperate
from such surgery — that time frame would be reasonable. For the smaller
variations on hepatic resection, such as a left hepatectomy, shorter windows of
time may be appropriate.
The administration of bevacizumab after radiofrequency ablation has not been
well studied. It is a less invasive procedure, although it does have potential
risks. A window of two to four weeks after radiofrequency ablation is probably
adequate, provided the patient had no undue complications.
 Track 16 Track 16
 DR LOVE: Would you discuss the dosing of bevacizumab? DR LOVE: Would you discuss the dosing of bevacizumab? |
 DR HURWITZ: In the first-line setting, a dose of 5 mg/kg every two weeks,
which is a dose intensity of 2.5 mg/kg per week, is the standard of care. Some
ongoing studies, mostly of capecitabine-based regimens with oxaliplatin, are
using three-week cycles of equivalent dose intensity. Those are 7.5 mg/kg
every three weeks, which is essentially the same dosing, particularly given the
long half-life of the drug. In the second-line setting, the dose is 10 mg/kg
every two weeks. DR HURWITZ: In the first-line setting, a dose of 5 mg/kg every two weeks,
which is a dose intensity of 2.5 mg/kg per week, is the standard of care. Some
ongoing studies, mostly of capecitabine-based regimens with oxaliplatin, are
using three-week cycles of equivalent dose intensity. Those are 7.5 mg/kg
every three weeks, which is essentially the same dosing, particularly given the
long half-life of the drug. In the second-line setting, the dose is 10 mg/kg
every two weeks.
An older study, the initial randomized Phase II trial (Kabbinavar 2003), evaluated
placebo versus 5 mg/kg versus 10 mg/kg. In that study, the
5 mg/kg dose was a little bit better. A number of disclaimers relate to potential
imbalances between the 5 and 10 mg groups. However, the 5 mg/kg dose
looked good enough to pursue in a Phase III study, and that dose is clearly
active. We know the safety profile of that drug in that setting.
Most patients have received bevacizumab in the first-line setting, so we see
very few reasons to give bevacizumab in the second-line setting. One can come up with a few scenarios, such as patients having been on a clinical trial
without bevacizumab or some complication that caused postponing the treatment.
If bevacizumab were truly to be used as a second-line agent, I would
use a 10 mg/kg dose. In a hybrid scenario, such as a delayed first-line setting, I
would use 5 mg/kg.
 Track 17 Track 17
 DR LOVE: What about the combination of cetuximab and bevacizumab
— where do you see that heading? DR LOVE: What about the combination of cetuximab and bevacizumab
— where do you see that heading? |
 DR HURWITZ: The issue of combining biologics is an interesting one. Right
now, preclinical biology suggests synergy when targeting the EGF and VEGF
axes. Interesting pilot data came from the BOND-2 trial, which looked at
cetuximab with bevacizumab versus cetuximab/bevacizumab and irinotecan
(Saltz 2005). DR HURWITZ: The issue of combining biologics is an interesting one. Right
now, preclinical biology suggests synergy when targeting the EGF and VEGF
axes. Interesting pilot data came from the BOND-2 trial, which looked at
cetuximab with bevacizumab versus cetuximab/bevacizumab and irinotecan
(Saltz 2005).
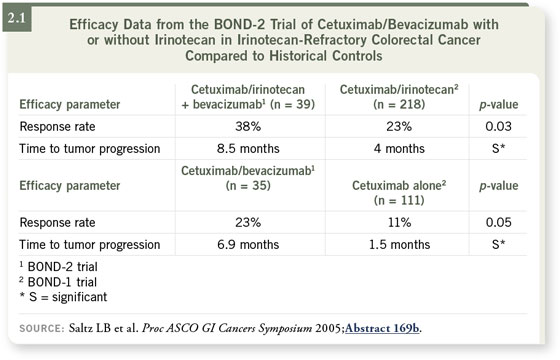
Both arms of that study appeared to do much better than the historical
controls of the BOND-1 trial (Cunningham 2004), which evaluated cetuximab
monotherapy or cetuximab with irinotecan (2.1). The response rates and
time to progression were a lot higher than most of us expected.
For both theoretical and practical reasons, I think the combination is highly
worthy of study. Two studies will address this question. The largest study will
be a GI Intergroup study of one chemotherapy regimen picked by the investigator
— either FOLFOX or FOLFIRI — with the addition of cetuximab,
bevacizumab or both (CALGB-C80405). An industry-sponsored study will
evaluate FOLFOX/bevacizumab with or without panitumumab, a monoclonal
antibody that is essentially a biological cousin of cetuximab.
 Track 20 Track 20
 DR LOVE: Previously, you mentioned the issue of capecitabine with
bevacizumab. Is there any reason to believe this combination would be
better or worse than bevacizumab with 5-FU? DR LOVE: Previously, you mentioned the issue of capecitabine with
bevacizumab. Is there any reason to believe this combination would be
better or worse than bevacizumab with 5-FU? |
 DR HURWITZ: My expectation is that the regimens would be comparable.
Large Phase III studies have been conducted, primarily in Europe, including a
FOLFOX versus CAPOX study with or without bevacizumab. This study has
completed accrual, and the toxicity results will be available in the next year.
Efficacy results will come shortly thereafter, but it may take a while for the
survival endpoint data to appear. DR HURWITZ: My expectation is that the regimens would be comparable.
Large Phase III studies have been conducted, primarily in Europe, including a
FOLFOX versus CAPOX study with or without bevacizumab. This study has
completed accrual, and the toxicity results will be available in the next year.
Efficacy results will come shortly thereafter, but it may take a while for the
survival endpoint data to appear.
In general, capecitabine has tended to look similar to 5-FU, including the
capecitabine/oxaliplatin versus infusional 5-FU/oxaliplatin data, without
bevacizumab, reported at ASCO this past year (Arkenau 2005; Sastre 2005).
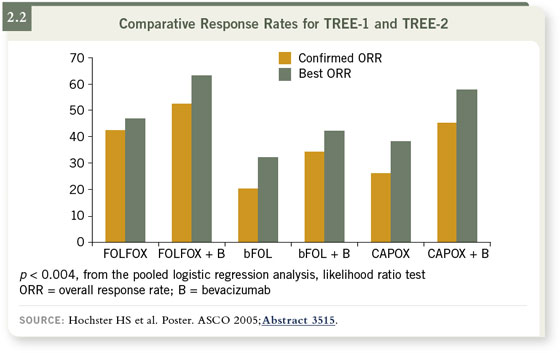
In addition, pilot data from our institution and the larger experience in
the TREE-2 study suggest that efficacy should be comparable between
capecitabine/oxaliplatin/bevacizumab and the FOLFOX regimen (2.2). That
comparability, though, has not yet been validated. As far as clinical management
goes, I would still consider an infusional 5-FU regimen with oxaliplatin
or irinotecan — FOLFOX or FOLFIRI — as the standard platform on which
to add bevacizumab.
For patients who are not candidates for pump therapy, the activity of
capecitabine with oxaliplatin and bevacizumab is significant enough that it
should be considered as a first-line option. The question of whether or not it’s
the best option will require the final results of this Phase III study.
Select publications
|

