You are here: Home: CCU 1 | 2006: Al B Benson III, MD

Tracks 1-8 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Allelic loss of chromosome 18q
and prognosis in colorectal
cancer |
| Track 3 |
ECOG-E5202: FOLFOX with or
without bevacizumab in
molecularly identified high-risk
Stage II disease |
| Track 4 |
ECOG-E5202: Eligibility criteria |
|
| Track 5 |
ECOG-E5202: Duration of
bevacizumab therapy |
| Track 6 |
ASCO treatment guidelines for
Stage II disease |
| Track 7 |
Current and potential future
strategies to select patients for
adjuvant therapy |
| Track 8 |
Alternative methods of 5-FU
administration |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: Can you discuss the background to ECOG trial 5202 in
patients with Stage II disease? DR LOVE: Can you discuss the background to ECOG trial 5202 in
patients with Stage II disease? |
 DR BENSON: A great deal of emphasis has been placed on laboratory
correlative work linked to outcome data from randomized clinical trials.
Correlative laboratory studies can be designed in several ways, although the
most typical evaluation to date is a retrospective analysis of patient tumor
specimens obtained from patients in clinical trials for which outcome data are
already available. DR BENSON: A great deal of emphasis has been placed on laboratory
correlative work linked to outcome data from randomized clinical trials.
Correlative laboratory studies can be designed in several ways, although the
most typical evaluation to date is a retrospective analysis of patient tumor
specimens obtained from patients in clinical trials for which outcome data are
already available.
One area that has been evaluated extensively — mostly in the retrospective
arena — is the phenomenon of 18q deletion, which has been associated with
a much poorer prognosis. Under the direction of Stan Hamilton’s laboratory,
ECOG examined tumor blocks from the primary tumors of individuals who
had entered one of two of the early Intergroup adjuvant trials, which were
5-FU based.
Unfortunately, because these are older trials, it was impossible to collect all
of the tumor specimens we needed. However, we did have sufficient tumor
specimens to conduct a retrospective analysis, which demonstrated that those patients who retain the 18q allele have a far better survivorship than those who
do not ( Jen 1994).
We also looked at microsatellite instability (MSI) in this series of patients, and
it appeared that patients with MSI and evidence of the TGF-beta mutation had
a superior survivorship compared to those who did not (Watanabe 2001).
In the cooperative group setting, randomized trials for patients with Stage III
disease who receive a 5-FU-based regimen report five-year survival rates near
65 percent. Among patients with 18q deletion — in other words, a poor-risk
group — five-year survival was only 50 percent. This correlates with survival
statistics for patients who had surgery alone. The implication is that the 5-FU-based
regimen had no impact on outcome.
On the other hand, those so-called good-risk patients who retained 18q or
had MSI with the TGF-beta mutation had a survival rate of 75 percent at five
years. What we don’t know in this population is whether this group would
have done just as well with surgery alone or if the chemotherapy had some
impact. We cannot answer those questions.
 Track 4-5 Track 4-5
 DR LOVE: How are the high- and low-risk patients identified in
trial 5202? DR LOVE: How are the high- and low-risk patients identified in
trial 5202? |
 DR BENSON: The laboratory correlative data were felt to provide enough
evidence to conduct a hypothesis-driven trial. The Intergroup elected to
evaluate patients’ primary tumor specimens for the 18q allele as well as for
MSI. The specimens are collected as close to the time of surgery as possible. DR BENSON: The laboratory correlative data were felt to provide enough
evidence to conduct a hypothesis-driven trial. The Intergroup elected to
evaluate patients’ primary tumor specimens for the 18q allele as well as for
MSI. The specimens are collected as close to the time of surgery as possible.
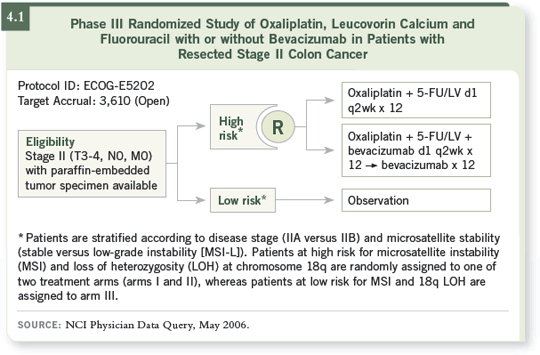
When the result is known for the Stage II patients who are asked to participate
in the trial, patients are assigned to either the high- or low-risk group. The
low-risk group will include those individuals who retain 18q. Our projected
survivorship for this group is nearly 90 percent. Of course, this trial will tell
us if we’re right or wrong in that assumption.
In the high-risk group, another suggestion based on retrospective data
indicated that a 5-FU/leucovorin regimen would not be likely to have a major
impact. Given the MOSAIC trial data implying that a worse prognostic group
tends to receive a greater benefit from FOLFOX (André 2004), it was elected
to incorporate FOLFOX into the randomization for the high-risk patients.
So the Intergroup strategy for both colon and rectal cancer is to randomly
assign these patients between FOLFOX and FOLFOX with bevacizumab. This
is what is being done for Stage II patients in the 5202 trial (4.1).
5202 is now active, and we encourage patients and physicians to participate
since it is really the first large-scale prospective randomized trial to determine
whether a molecular parameter in colon cancer can aid us in making treatment
decisions. In addition, we will be storing tissue to evaluate other potential
prognostic and predictive markers once we have outcome data available.
Select publications
|

