You are here: Home: CCU 1 | 2006: Peter C Enzinger, MD

Tracks 1-20 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Comparison of NSABP-C-07 and
MOSAIC adjuvant trial outcomes |
| Track 3 |
Preservation of oxaliplatin dose
intensity during adjuvant therapy |
| Track 4 |
Use of calcium and
magnesium for oxaliplatin-associated
neuropathy |
| Track 5 |
Dose attenuation or
discontinuation for oxaliplatin-associated
neuropathy |
| Track 6 |
Probability of residual
neuropathy after oxaliplatin-containing
chemotherapy |
| Track 7 |
X-ACT adjuvant trial: Capecitabine
versus Mayo Clinic 5-FU regimen |
| Track 8 |
Selecting appropriate patients
to receive capecitabine as
adjuvant therapy |
| Track 9 |
Dosing capecitabine in the
adjuvant and metastatic settings |
| Track 10 |
Differences in North American
and European tolerance to
capecitabine dosing |
|
| Track 11 |
Evaluating patients for
selection of initial therapy in the
metastatic setting |
| Track 12 |
Bevacizumab-associated
toxicities |
| Track 13 |
Potential mechanisms of action
of bevacizumab |
| Track 14 |
Selection of second-line
therapy after progression on a
bevacizumab-containing regimen |
| Track 15 |
Side effects and tolerability of
bevacizumab and cetuximab |
| Track 16 |
Rationale for evaluating bevacizumab
in adjuvant clinical trials |
| Track 17 |
CALGB-C80405: Cetuximab and/or bevacizumab and FOLFOX or
FOLFIRI in the metastatic setting |
| Track 18 |
Therapeutic approach to patients
with Stage II disease |
| Track 19 |
Clinical trials evaluating benefit
of adjuvant chemotherapy in
Stage II disease |
| Track 20 |
Laparoscopic colectomy and
number of nodes removed |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: Would you discuss the NSABP-C-07 and MOSAIC trials? DR LOVE: Would you discuss the NSABP-C-07 and MOSAIC trials? |
 DR ENZINGER: Unlike the MOSAIC study (de Gramont 2003; André 2004),
the FLOX — or NSABP-C-07 — study (Wolmark 2005) specifically looked
at a bolus regimen of 5-FU and leucovorin with or without oxaliplatin. In a sense, it was the traditional Roswell Park regimen with oxaliplatin added to
every other treatment. DR ENZINGER: Unlike the MOSAIC study (de Gramont 2003; André 2004),
the FLOX — or NSABP-C-07 — study (Wolmark 2005) specifically looked
at a bolus regimen of 5-FU and leucovorin with or without oxaliplatin. In a sense, it was the traditional Roswell Park regimen with oxaliplatin added to
every other treatment.
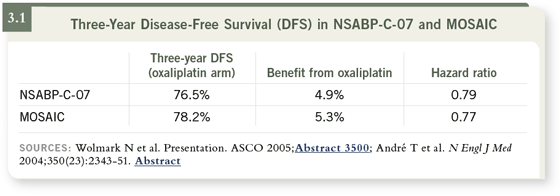
The accrual for the trial took a little bit longer than that of the MOSAIC
study, and the results were reported at a later date. NSABP-C-07 investigators
ultimately came to the conclusion that oxaliplatin adds a 4.9 percent absolute
benefit when added to 5-FU and leucovorin therapy, almost the same absolute
three-year benefit that was found in the MOSAIC study (3.1).
The question is whether we need to give infusional 5-FU or whether we
can continue with bolus dosing of 5-FU. Ultimately, I think the infusional
5-FU schedules have lower overall toxicity, but the dose of oxaliplatin in the
MOSAIC study was higher, so patients had a higher incidence of neuropathy.
In NSABP-C-07 (Wolmark 2005), a 10 percent difference in Grade III and
Grade IV toxicities was evident between the Roswell Park regimen (FU/LV)
and the same regimen plus oxaliplatin (FLOX). Seven percent of that difference
is due to neuropathy. So the difference in overall toxicity is really only
three percent.
The ultimate conclusion is that NSABP-C-07 is a confirmatory trial. It
confirms the benefit of oxaliplatin, and it offers oncologists an alternative way
of administering this agent in combination with 5-FU and leucovorin, which
is reassuring.
I would like to see the overall survival advantage for both trials, and that will
probably become evident as deaths increase. When we look at the number of
patients who are dying and the patients who are having recurrences, we see
that the number of patients with recurrences is increasing.
We’re beginning to see a trend in that direction. My suspicion is that it’s going
to take some time before we actually see an overall survival advantage in
either one of these trials.
 Track 5 Track 5
 DR LOVE: In what situation would you discontinue oxaliplatin due to
neurotoxicity? I hear people talk about discontinuation prior to the development
of functional impairment. Is that your approach? DR LOVE: In what situation would you discontinue oxaliplatin due to
neurotoxicity? I hear people talk about discontinuation prior to the development
of functional impairment. Is that your approach? |
 DR ENZINGER: Yes, although I think the more interesting question is when to
start attenuating the dose. Do you push the patient to functional impairment
using the full dose, or do you start attenuating early or drop the oxaliplatin as
in the OPTIMOX trial (de Gramont 2004)? DR ENZINGER: Yes, although I think the more interesting question is when to
start attenuating the dose. Do you push the patient to functional impairment
using the full dose, or do you start attenuating early or drop the oxaliplatin as
in the OPTIMOX trial (de Gramont 2004)?
There are many ways of doing this in metastatic disease, and I don’t think
there’s one right or wrong way. With many other therapies available, giving
treatment for a certain period of time and then switching to something else
before these neuropathies arise may be the better approach.
Colleagues who treat lung and breast cancer utilize a set number of cycles, or
they treat for a set period of time. It’s really only with the GI malignancies,
because our patients have such a poor prognosis, that we treat continuously.
We probably should begin to consider adopting more of a breast or lung cancer
type of approach. In part, I believe that patients will have a better quality of
life using that kind of approach.
 DR LOVE: FOLFOX in the metastatic setting is very different from using it in
the adjuvant setting. In the adjuvant setting, how many patients discontinue
the drug? Generally, when is it stopped? DR LOVE: FOLFOX in the metastatic setting is very different from using it in
the adjuvant setting. In the adjuvant setting, how many patients discontinue
the drug? Generally, when is it stopped?
 DR ENZINGER: I almost always have to attenuate the oxaliplatin toward the
end of the treatment. I need to attenuate oxaliplatin starting around the eighth
or ninth treatment in 80 or 90 percent of all patients. I’ve had to drop oxaliplatin
completely in 10 percent. DR ENZINGER: I almost always have to attenuate the oxaliplatin toward the
end of the treatment. I need to attenuate oxaliplatin starting around the eighth
or ninth treatment in 80 or 90 percent of all patients. I’ve had to drop oxaliplatin
completely in 10 percent.
 Track 6 Track 6
 DR LOVE: When patients are about to start receiving FOLFOX for the
first time and they ask you about the chances of having some type of
residual neuropathy, how do you respond? DR LOVE: When patients are about to start receiving FOLFOX for the
first time and they ask you about the chances of having some type of
residual neuropathy, how do you respond? |
 DR ENZINGER: I tell them that we don’t know whether or not this will occur.
The data right now are out to 18 months. But I try to reassure the patient in
the sense that the neuropathy — at least according to the MOSAIC study — is
very mild. So I tell them that about 24 percent of patients have some residual
neuropathy, but only 0.5 percent have debilitating neuropathy. I think those
odds are pretty good. DR ENZINGER: I tell them that we don’t know whether or not this will occur.
The data right now are out to 18 months. But I try to reassure the patient in
the sense that the neuropathy — at least according to the MOSAIC study — is
very mild. So I tell them that about 24 percent of patients have some residual
neuropathy, but only 0.5 percent have debilitating neuropathy. I think those
odds are pretty good.
Most patients have experienced some tingling or mild tingling, and that’s what
I tell my patients to expect. I don’t believe that they’re going to be left with
any debilitating neuropathies. I think the risk for another adverse event is
much higher than for long-term debilitating neuropathy.
 Track 7 Track 7
 DR LOVE: Another major trial that’s had a big impact on clinical
decision-making is the X-ACT study, which evaluated capecitabine versus
5-FU (Cassidy 2004; Twelves 2005a, 2005b). Can you summarize that
study and discuss your interpretation of the findings? DR LOVE: Another major trial that’s had a big impact on clinical
decision-making is the X-ACT study, which evaluated capecitabine versus
5-FU (Cassidy 2004; Twelves 2005a, 2005b). Can you summarize that
study and discuss your interpretation of the findings? |
 DR ENZINGER: The X-ACT study evaluated the FDA-approved dose of
capecitabine, which is 2,500 mg/m2 daily for 14 days straight every three
weeks for six months. The standard treatment arm in that trial received the
Mayo Clinic 5-FU regimen. DR ENZINGER: The X-ACT study evaluated the FDA-approved dose of
capecitabine, which is 2,500 mg/m2 daily for 14 days straight every three
weeks for six months. The standard treatment arm in that trial received the
Mayo Clinic 5-FU regimen.
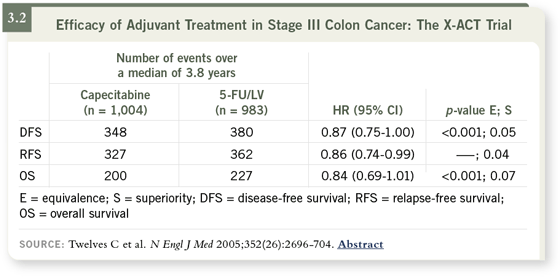
The study was powered to detect relatively small differences in survival and
also to look at equivalence between the arms. The three-year results evaluated
relapse-free survival, disease-free survival and overall survival.
At the three-year analysis, we saw that the regimen of capecitabine was at
least equivalent to the Mayo Clinic 5-FU regimen (3.2). In fact, there was a
statistically significant improvement in relapse-free survival, and disease-free
survival was right on the edge of significant improvement.
I think overall survival will also show an improvement with further follow-up.
We’re seeing the same thing here that we’re seeing in the metastatic
disease setting — that capecitabine is probably slightly better than what we’ve
observed with bolus 5-FU.
 Track 8 Track 8
 DR LOVE: How do you determine which patients are candidates for
capecitabine in the adjuvant setting? DR LOVE: How do you determine which patients are candidates for
capecitabine in the adjuvant setting? |
 DR ENZINGER: The goalpost for adjuvant treatment has been changed since
X-ACT was initiated. Most doctors are now recommending FOLFOX, particularly
for patients who are at higher risk for recurrence. So the question now
is where capecitabine fits into this whole category. DR ENZINGER: The goalpost for adjuvant treatment has been changed since
X-ACT was initiated. Most doctors are now recommending FOLFOX, particularly
for patients who are at higher risk for recurrence. So the question now
is where capecitabine fits into this whole category.
We know capecitabine had a very nice toxicity profile in the head-to-head
comparison with the Mayo Clinic regimen (Twelves 2005b). Toxicities across
the board were less than those associated with the Mayo Clinic regimen,
except for hand-foot syndrome, with which 17 percent of patients had severe
Grade III to Grade IV toxicity.
But the reality is that this will occur during the first cycle. Then you attenuate
treatment, and typically, patients will do fine for the rest of the adjuvant
course.
Looking at the X-ACT data, a subgroup analysis suggests that patients with
N1 disease did better than those with N2 disease, which makes sense to me
and would actually fit in nicely with the FOLFOX paradigm.
In my mind, I would say that patients with N2 disease, the patients who have
higher risk for recurrence, really should not even be given an option. Those
patients should be pushed towards FOLFOX.
Patients who have a lower risk for recurrence, perhaps those with N1 disease,
and patients who wish to avoid the neuropathies may actually be good candidates
for capecitabine. So perhaps the patients with Stage IIIA disease would
do well with capecitabine.
In my own practice, patients with high-risk Stage III disease receive
FOLFOX. With low-risk patients, I have an intensive conversation about the
risks and benefits of FOLFOX versus the Roswell Park 5-FU regimen
versus capecitabine.
 DR LOVE: What are the situations in which you’d use the Roswell Park
regimen instead of capecitabine? DR LOVE: What are the situations in which you’d use the Roswell Park
regimen instead of capecitabine?
 DR ENZINGER: In my opinion, the Roswell Park regimen is well suited for
an elderly patient who needs the social support associated with coming to the
office and seeing the nurses. Often, they enjoy the stimulation of coming for
a visit. DR ENZINGER: In my opinion, the Roswell Park regimen is well suited for
an elderly patient who needs the social support associated with coming to the
office and seeing the nurses. Often, they enjoy the stimulation of coming for
a visit.
Capecitabine is a therapy for a business professional, for somebody who
believes that adjuvant therapy gets in their way and who wants to have the
least number of interruptions possible.
Now we really haven’t seen any direct comparisons between Roswell Park and
capecitabine. Capecitabine has always been measured against the Mayo Clinic
regimen, so I don’t know how a toxicity analysis between these other two
would work out.
I suspect that Roswell Park would be associated with more diarrhea, more
hematologic toxicity and stomatitis, but the difference would be less than
when compared with the Mayo Clinic regimen.
 DR LOVE: What about patients with Stage II disease? DR LOVE: What about patients with Stage II disease?
 DR ENZINGER: I think patients with Stage II disease sometimes have a higher
risk for recurrence than those with Stage III disease. A patient with Stage IIB
colorectal cancer, in my opinion, may have a higher risk for recurrence than
someone with Stage IIIA disease, and again, that’s where I’d consider all three
options. DR ENZINGER: I think patients with Stage II disease sometimes have a higher
risk for recurrence than those with Stage III disease. A patient with Stage IIB
colorectal cancer, in my opinion, may have a higher risk for recurrence than
someone with Stage IIIA disease, and again, that’s where I’d consider all three
options.
I tend to push patients towards FOLFOX if they have tumors that have perforated.
 Track 16 Track 16
 DR LOVE: The design of randomized trials, both in the metastatic and
the adjuvant setting, is becoming a lot more complicated because of all
the new agents. What are some of the current research questions that you
think are most interesting? DR LOVE: The design of randomized trials, both in the metastatic and
the adjuvant setting, is becoming a lot more complicated because of all
the new agents. What are some of the current research questions that you
think are most interesting? |
 DR ENZINGER: One of the most interesting questions in the adjuvant setting
is the role of bevacizumab. It is now shown to be of significant benefit in the
metastatic disease setting. Can we duplicate that in the adjuvant setting? DR ENZINGER: One of the most interesting questions in the adjuvant setting
is the role of bevacizumab. It is now shown to be of significant benefit in the
metastatic disease setting. Can we duplicate that in the adjuvant setting?
People have argued that we’re destroying isolated cancer cells in the adjuvant
setting, and isolated cancer cells don’t need vasculature — they don’t need to
recruit oxygen. However, I believe our imaging capabilities are suboptimal.
We’re able to detect cancer nodules that are approximately one centimeter in
size — it’s quite clear that we’re not able to detect a cancer nodule less than
0.5 centimeters in size, even in the best-case scenarios. A one-centimeter
nodule is significantly larger than the one- to two-millimeter nodule that is
the size at which we think the angiogenic switch is being thrown.
If a patient has a tumor nodule between one and 10 millimeters, I believe
that’s where bevacizumab will probably have its greatest impact. Not only will
it have an anti-angiogenic effect, but it’ll also promote drug delivery into these
fairly large conglomerates of tumor cells. We’re talking about many millions of
cells in these small tumor nodules.
In designing these trials, I would stratify and look at patients who have
poor-risk Stage III disease — the patients who probably have residual tumor
nodules, because I think bevacizumab may actually exert its greatest impact
or activity in patients with macroscopic residual disease that we just can’t see
— not in microscopic residual disease.
 Track 17 Track 17
 DR LOVE: Can you discuss the upcoming Intergroup trial that will
evaluate combined biologic therapy in metastatic disease? DR LOVE: Can you discuss the upcoming Intergroup trial that will
evaluate combined biologic therapy in metastatic disease? |
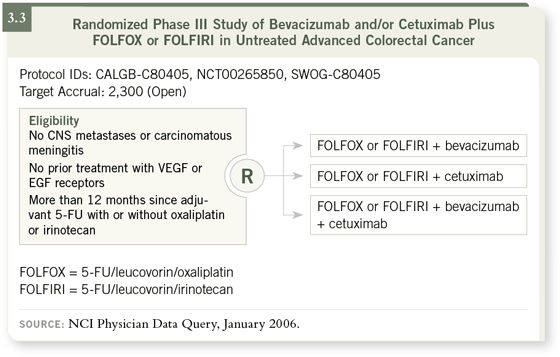
 DR ENZINGER: In the upcoming Intergroup study, patients will be randomly
assigned to standard chemotherapy (FOLFOX or FOLFIRI) with standard
treatment (bevacizumab) or with experimental treatment that includes cetuximab
or cetuximab with bevacizumab (3.3). Based on the data we’ve seen
from the CBI trial (Saltz 2005), many of us expect the arm receiving both
biologic agents to win. DR ENZINGER: In the upcoming Intergroup study, patients will be randomly
assigned to standard chemotherapy (FOLFOX or FOLFIRI) with standard
treatment (bevacizumab) or with experimental treatment that includes cetuximab
or cetuximab with bevacizumab (3.3). Based on the data we’ve seen
from the CBI trial (Saltz 2005), many of us expect the arm receiving both
biologic agents to win.
The dilemma is that this will be very expensive therapy, but I think that it’s
going to be very interesting. I think many of us are already tempted to throw
everything at these patients — particularly the patients who are at high risk.
However, use of both biologics raises financial costs and also toxicity costs.
Select publications
|

