You are here: Home: CCU 1 | 2006: Editor's Note

Like all of our audio programs, this issue of Colorectal Cancer Update is stuffed
like a kishka with scientific content. Herb Hurwitz updates us on the evolving
and very encouraging short- and long-term safety data on the anti-VEGF
agent bevacizumab; Peter Enzinger comprehensively reviews recent data
on adjuvant chemotherapy, particularly trials of oxaliplatin regimens like
FOLFOX and FLOX (I prefer lox) and studies of capecitabine, either alone
or with oxaliplatin; and Al Benson discusses the background and design of
ECOG trial 5202, a critical study evaluating FOLFOX alone or with bevacizumab
in Stage II tumors that a central lab at MD Anderson designates as
higher risk based on microsatellite instability and 18q deletions.
There is also a Cracker Jack®-like special prize included with this program, a
report on an exciting patient education project we recently conducted on 150
people with colorectal cancer who reacted to an audio program outlining the
risks and benefits of adjuvant chemotherapy.
As discussed on the last issue of this series, our findings provide a number
of interesting insights about patient perspectives on this disease. To that end,
the enclosed monograph includes a comprehensive look at the survey results
and a CD with the 50-minute audio interview with John L Marshall, MD
that formed the basis of the survey. Many of Dr Marshall’s comments are also
excerpted in the print report.
As this note is being composed, our CME group is preparing to travel to
San Francisco for the third annual ASCO GI symposium, where we will
present a poster outlining many of our major findings from this project. We
look forward to onsite and “virtual” feedback regarding this initial foray into
patient education and our plan to pilot a “boxed set” of six CDs in 2006 on
a variety of patient education issues related to adjuvant systemic therapy for
colon cancer.
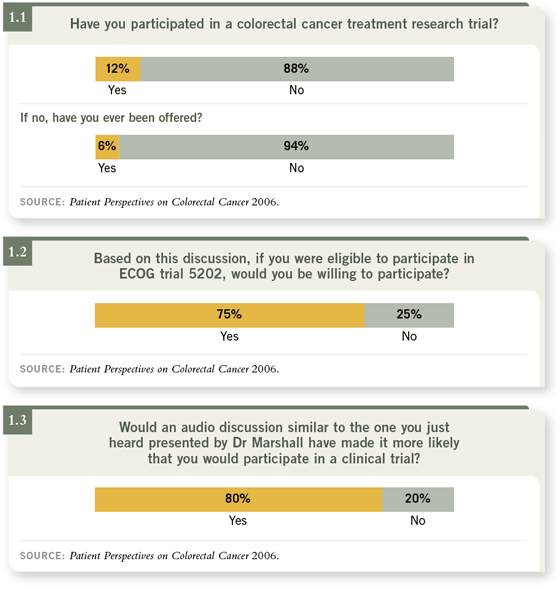
Of all the fascinating nuggets to come out of this initiative, perhaps my
favorite relates to ECOG trial 5202, which randomly assigns patients with
higher-risk Stage II tumors to FOLFOX alone or with bevacizumab. Based on
Dr Marshall’s description of this study, 75 percent of the participants would be
willing to enter the study if eligible (1.1, 1.2, 1.3).
Seventy-five percent is an impressive fraction and is far more than the
estimated two to three percent of cancer patients nationally who enter clinical
trials. Yet, perhaps we should not be too surprised by this finding. Trial 5202
offers participants not only a chance to move the field forward and protect
the health of future generations but also the unique opportunity to have their
tumor tissue analyzed by one of the best labs in the country. Based on those
findings, participants can potentially receive a relatively nontoxic therapeutic
agent (bevacizumab) that would not be available off protocol.
Seventy-five percent. Let’s tap into this signal and get trials like 5202 and its
siblings, NSABP-C-08 and AVANT, done — and done soon. We need more
good stuff to talk about on our CME programs in the future, and nothing
would be more interesting and encouraging than a trastuzumab-like step
forward in adjuvant therapy for colon cancer.
— Neil Love, MD
NLove@ResearchToPractice.net
January 30, 2006
|

