You are here: Home: CCU 4 | 2004: Robert A Wolff, MD
| Robert A Wolff, MD |
EDITED COMMENTS |
 Continuous infusion 5-FU versus capecitabine in rectal cancer Continuous infusion 5-FU versus capecitabine in rectal cancer
In the adjuvant setting, when infusional 5-FU rather than bolus 5-FU is combined with radiation therapy, disease-free and overall survival are improved. Infusional 5-FU is a better radiosensitizing agent. The advantage of infusional 5-FU with radiation therapy is probably more of a systemic rather than a local control benefit.
An Intergroup trial published in the New England Journal of Medicine demonstrated a trend toward better local control using infusional 5-FU compared to bolus 5-FU. That study is proof of the principle that infusional 5-FU is a superior treatment modality when combined with radiation therapy.
Capecitabine is an interesting alternative to infusional 5-FU for several reasons. With infusional 5-FU, catheter-related problems can develop, such as thrombosis and infection. Additionally, patients are required to carry an ambulatory pump. When the pump is on for a couple of weeks it’s no big deal, but generally by the fifth week of radiation therapy, patients are tired of it. Capecitabine is a nicer route of administration.
Additionally, capecitabine is a prodrug, and it has to be converted to 5-FU at the intracellular level. One of the enzymes responsible for that conversion is thymidine phosphorylase (TP), which is expressed in higher concentrations in the rectal mucosa.
At the biological level, that may mean that the rectum, the rectal mucosa and the tumor cells have a higher intracellular concentration of 5-FU, leading to both an active cytotoxic benefit and more radiosensitization. We have therefore been interested in evaluating capecitabine as a radiosensitizer compared to infusional 5-FU.
In the future, we will combine capecitabine with bevacizumab. That trial is not yet open, but we will pursue not only conventional cytotoxic agents with radiation but also utilize biologic agents such as bevacizumab for this group of patients.
When we evaluated capecitabine plus irinotecan (CAPIRI), we limited accrual to patients with metastatic rectal cancer. They were a challenging group not only because they have metastatic disease but also because of concern about local control issues. We prefer to spare these patients surgery, but we obviously have to worry about obstructive symptoms, pelvic symptoms and the like. Many of our trials — particularly of novel chemoradiation programs — are limited to patients with metastatic rectal cancer as opposed to resectable disease.
Dose and schedule of capecitabine utilized with preoperative radiation therapy
For preoperative radiation therapy, we’ve used capecitabine — mostly on protocol — in a seven-day continuous treatment using approximately 1,650 mg/m2 per day throughout the course of radiation therapy at 50.4 Gray.
It is confusing that in some of our preoperative protocols, the radiotherapy was changed. People are beginning to evaluate concomitant boost radiation therapy, particularly toward the end of radiation therapy. The San Antonio group reported on concomitant boost radiation therapy for rectal cancer with twice daily radiation on the first and last few days of radiation therapy. We’ve evaluated boost radiation therapy only during the last few days.
The other way you can administer capecitabine is five days in a row with the weekends off. In my experience, that is a little easier because it carries less risk of hand-foot syndrome and less perianal skin irritation and diarrhea. I don’t think those schedules result in substantial differences. In terms of efficacy, I doubt if a direct comparison between a seven-day and five-day schedule of capecitabine would result in a meaningful difference in pathological CR rates, tolerance, etcetera.
Potential explanations for the negative results of CALGB-C89803
I was surprised that this adjuvant study comparing IFL (irinotecan, bolus 5-FU and leucovorin) versus 5-FU/leucovorin was negative (1.1). One potential explanation is synergy — a concept that is bandied about a lot among oncologists. I don’t think there’s any question that the combination of 5-FU and oxaliplatin is truly synergistic. Most clinicians and laboratory-based physicians argue that 5-FU/ leucovorin and irinotecan — at least with 5-FU/leucovorin as a bolus — is only additive. That is the simplest explanation why IFL didn’t really gain much ground in the adjuvant setting. Clones that are resistant to 5-FU/leucovorin may be somewhat effected by irinotecan but the data indicate the effect is not significant.
If you pick the right patient population or if you use infusional 5-FU with irinotecan, which may have more of a positive interaction, would you get the same result? I don’t know. Infusional 5-FU and irinotecan or capecitabine and irinotecan may actually be good combinations, but I’m not certain how much that will be pursued. Cooperative groups will need to decide whether or not to investigate combination chemotherapy with irinotecan because we already have a very nice combination in FOLFOX4 that has demonstrated superiority.
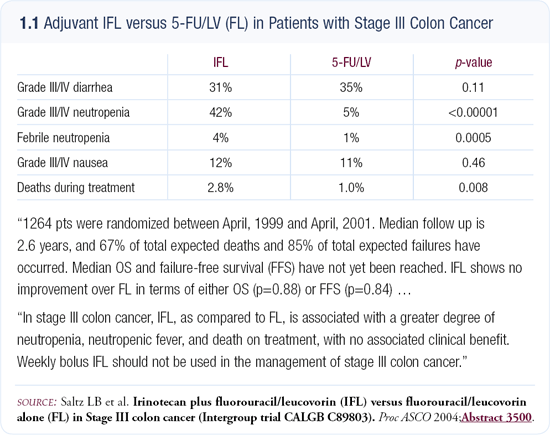
Another potential explanation for the negative outcome is that patients were not able to receive enough treatment with IFL due to toxicity. A few more treatmentrelated deaths occurred in the IFL arm of that study, but it doesn’t appear that the toxicity issues were enough to discontinue therapy for those patients.
Phase II trial of 5-FU/leucovorin with or without bevacizumab
In a Phase II trial evaluating 5-FU/leucovorin with or without bevacizumab in patients previously treated for metastatic disease, bevacizumab resulted in higher response rates, improved time to progression and a trend toward improved survival. This trial actually launched the Phase III trial that was presented last year at ASCO, which demonstrated an advantage to adding bevacizumab to IFL. Those results were striking and were a tremendous boost to the morale of many medical oncologists. In preliminary analyses, it appears that 5-FU/leucovorin and bevacizumab are at least as good as 5-FU/leucovorin and irinotecan. It’s fascinating that substituting a biological agent for a third chemotherapeutic drug can produce similar efficacy.
Select Publications
 |
| Dr Wolff is an Associate Professor of Medicine and Deputy Chairman for Clinical Affairs at the University of Texas MD Anderson Cancer Center’s Department of Gastrointestinal Medical Oncology in Houston, Texas. |
|

