You are here: Home: CCU 4 | 2004: Philip J Gold, MD
| Philip J Gold, MD |
EDITED COMMENTS |
 SWOG-S0303: Phase III randomized trial comparing FOLFOX6 to CAPOXwith or without bevacizumab SWOG-S0303: Phase III randomized trial comparing FOLFOX6 to CAPOXwith or without bevacizumab
We do not yet have head-to-head comparisons between capecitabine and infusional 5-FU when administered in combination with irinotecan or oxaliplatin. Those studies are all in development or just opening.
Our next effort will be SWOG-S0303 (2.1), a very large Intergroup trial that will randomly assign patients to FOLFOX6 (infusional 5-FU, leucovorin and oxaliplatin) or CAPOX (capecitabine and oxaliplatin).
A second randomization will then assign the patients to bevacizumab or placebo.
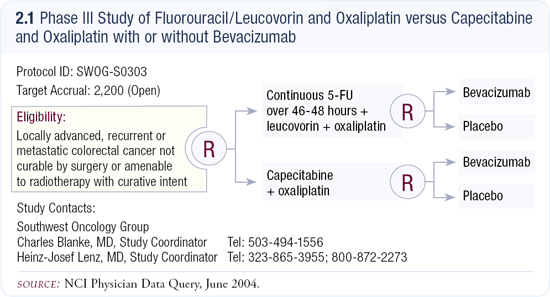
That trial will answer two questions: (1) Can capecitabine substitute for infusional 5-FU as first-line therapy in patients with metastatic colorectal cancer, and (2) What does the addition of a novel biologic agent contribute?
Rationale for the study design of SWOG-S0303
Based on preliminary data from N9741, FOLFOX (infusional 5-FU, leucovorin and oxaliplatin) was selected as the standard arm — although it was modified to FOLFOX6, which eliminates the day-two visit. In Europe, FOLFOX6 has been shown to be superior to FOLFOX4. Because of the drive to incorporate capecitabine-containing regimens, CAPOX became the other chemotherapy arm.
As reported at ASCO 2003, the addition of bevacizumab to IFL improved outcomes compared to IFL alone. The response rates, median survival times and times to progression for FOLFOX4 and IFL plus bevacizumab are superimposable.
We’re curious as to whether adding bevacizumab to an oxaliplatin-containing regimen will provide improvement similar to that seen when bevacizumab was added to IFL. ECOG-3200 is evaluating bevacizumab plus an oxaliplatincontaining regimen as second-line therapy.
If that trial demonstrates an advantage for the addition of bevacizumab, it is my hunch that all patients enrolled in SWOG-S0303 will receive bevacizumab, and the second randomization will be eliminated.
Capecitabine versus infusional 5-FU
Capecitabine may be more beneficial than infusional 5-FU, particularly if we can identify patients with high levels of intratumoral thymidine phosphorylase (TP) — the rate-limiting step for the activation of capecitabine. Patients with high levels of TP and lower levels of thymidylate synthase may have a higher probability of responding to capecitabine.
Compared to bolus 5-FU, capecitabine has a higher response rate and less toxicity, but equivalent survival. However, we don’t yet have a head-to-head comparison between infusional 5-FU and capecitabine. Based on trials conducted mainly in Europe, my hunch is that they will be virtually identical.
For FOLFOX4 or FOLFOX6, the pooled response rate is about 45 percent, the time to progression is approximately eight months and the median survival is approximately 19 months. According to pooled data — admittedly not a scientific method — similar results are reported for CAPOX and CAPIRI. While we await results from large Phase III comparative trials, the Phase II data suggest a similar level of activity for capecitabine-containing regimens compared to infusional 5-FU-containing regimens.
Potential utility of capecitabine-containing regimens for patients with metastatic disease
With capecitabine-containing regimens, we can remove the central line and eliminate the discomfort and potential complications it causes in patients receiving infusional 5-FU. Capecitabine is more convenient for patients, extremely well-tolerated and less expensive. We treated many patients with CAPIRI as a study protocol, and we were so pleased with the toxicity and efficacy results that it has become an off-study option.
SWOG-S0030: Phase II trial of capecitabine in elderly patients
A Phase II trial being conducted by SWOG (SWOG-S0030) will evaluate single-agent capecitabine in patients who are 70 years of age or older. It is a new trial that will accrue approximately 60 elderly patients. If I do not believe an elderly patient will tolerate oxaliplatin or irinotecan, I use single-agent capecitabine as a nonprotocol therapy.
Phase III trial of bevacizumab plus IFL
This was the most positive Phase III colon cancer trial for metastatic disease ever reported, and the first validation that an antiangiogenic agent, bevacizumab, may be effective in treating human cancer. In terms of efficacy, three numbers impressed me: the improvement in the response rate, time to progression and survival.
We enrolled about eight patients in that trial and encountered minimal additive toxicity from the bevacizumab. Hypertension is probably the most likely toxicity, but the incidence of significant hypertension was very modest. Six cases of GI perforation were reported, of which almost all were related to an underlying inflammatory state — peptic ulcer disease or diverticular disease. Epistaxis is another toxicity associated with bevacizumab; however, it usually stops spontaneously, and we did not encounter a problem in our limited number of patients.
Select Publications
 |
| Dr Gold is the Director of Clinical Research and Program Leader of GI Oncology at the Swedish Cancer Institute in Seattle, Washington. |
|

