You are here: Home: CCU 4 | 2004: George A Fisher, MD, PhD
| George A Fisher, MD, PhD |
EDITED COMMENTS |
 Capecitabine plus radiation therapy for the treatment of rectal cancer Capecitabine plus radiation therapy for the treatment of rectal cancer
Four Phase I studies and one relatively small Phase II trial have compared different capecitabine dosing schedules in combination with radiation. These were not always rectal cancer studies, but all of them showed that this combination was reasonably safe to administer.
The utilized schedules varied from every day to Monday through Friday to two weeks on, one week off. For convenience, we chose a Monday through Friday schedule to match our continuous Monday through Friday infusion regimen.
That way, patients received capecitabine every day they received radiation therapy. We used 1,000 mg/m2 twice daily, which was remarkably well-tolerated for the four or five weeks of radiation.
A small Phase II trial showed a pathologic complete response rate comparable to what we have seen with continuous infusion 5-FU. I cannot compare apples and oranges, but at least it is in the same ballpark, and capecitabine is a lot easier to administer. I think this is really a convenience issue. I don’t believe the thymidine phosphorylase upregulation data. I think it makes a nice pharmacologic argument as to why capecitabine should be superior, but I would not be disappointed if that data were all wrong. It would be exciting, however, if that data were true because the best way of taking advantage of that would be with radiation.
Utilization of capecitabine versus infusional 5-FU
I am not a big fan of bolus 5-FU. In fact, I am not sure there is any role for it in patients with metastatic disease. I believe capecitabine will be nearly as good as infusional 5-FU, but I don’t know that it needs to be identical. I suspect that it will be similar enough and convenient enough to be widely used. I think the biggest obstacles are financial ones — people with copays or substantial out-of-pocket expenses for oral drugs and oncologists who feel threatened that they will lose revenue without bolus 5-FU. However, capecitabine is clearly an easier treatment. I have heard many people voice concerns about noncompliance, but I don’t think those issues are pertinent. No population is more compliant than cancer patients. And frankly, if I give patients pills and tell them that they are good for their cancer, and they do not take those pills, I’ll respect that.
Response of the primary versus metastatic lesion to FOLFOX
It’s difficult to measure the actual size of primary lesions with CT assessment. When we measure them in terms of symptoms, I believe primary tumors tend to respond better than distant metastatic sites. Because flow through the lumen is a function of the radius cubed, minimal shrinkage or a minor response may result in major symptom improvement. I think, symptomatically, FOLFOX is actually a good therapy. We have known for years that neoadjuvant chemotherapy for obstructive esophageal lesions provides relief of dysphagia in approximately 80 percent of patients; however, we see response rates of only 30, 40 or 50 percent. I think when you’re looking at symptoms of a primary lesion, you get good relief, but the actual response rate is hard to measure, so I can’t tell you that they respond exactly the same way.
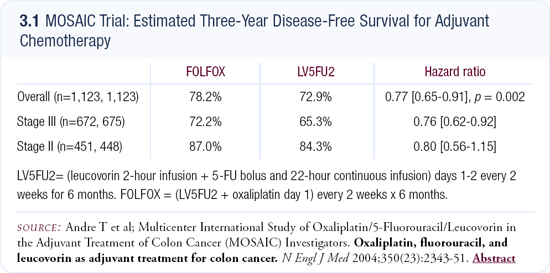
Impact of the MOSAIC adjuvant trial on clinical practice
The MOSAIC trial (3.1) is really shifting the sands of adjuvant therapy for colorectal cancer, particularly in light of the negative CALGB study, which showed that irinotecan and 5-FU combined were not better than 5-FU alone. The questions that remain are: How much better is adjuvant FOLFOX? Is the toxicity worth any incremental improvement? And will that incremental improvement hold up in terms of overall survival?
Certainly the toxicity seems tolerable. In the MOSAIC trial, about 18 percent of the participants had Grade III neuropathy during or shortly after the study. At one-year follow-up, that decreased to one percent. Grade III neuropathy is no fun, but patients have been living with cisplatin neurotoxicity for years. I think adjuvant FOLFOX is finding believers, not only in academic circles but also in the community. In particular, it’s being used for young patients with high-risk Stage III disease.
Phase III trial results of bevacizumab in combination with bolus IFL
When irinotecan was approved in combination with bolus 5-FU/leucovorin (IFL) based on a 2.2-month median survival advantage compared to bolus 5-FU/ leucovorin, it was definitely an incremental advance. However, I thought it was a bit over the top for the FDA to make its unprecedented statement that this regimen was the standard of care. The data for bevacizumab in combination with bolus IFL data blew that away with an approximately 4.8-month improvement in median survival, a higher response rate and no increase in toxicity. The bevacizumab plus IFL trial was very well executed and had all the right endpoints.
Proposed NSABP trial: Adjuvant FOLFOX with or without bevacizumab
The original trial randomly assigned patients to FLOX, FOLFOX or CAPOX, with a second randomization to bevacizumab or placebo. That trial design was going to require approximately 5,500 patients, and it was decided to ask only the bevacizumab question, which may be the more important question.
This gets back to the philosophical question of which data are necessary to change clinical practice. In the ovarian cancer community, carboplatin in combination with paclitaxel was adopted long before GOG decided to conduct a Phase III trial. With only good reproducible Phase II studies, a switch to carboplatin in combination with paclitaxel occurred for the treatment of patients with ovarian cancer. It’s a bit disingenuous of the intellectual community to say, “You can’t use capecitabine in combination with oxaliplatin but must use what’s been shown to be the standard of care.” If the standard of care is FOLFOX4, then why are they using FOLFOX6 and modified FOLFOX6? Those are just shades of gray.
Select Publications
 |
| Dr Fisher is an Assistant Professor of Medicine at Stanford University School of Medicine and Director of Oncology Clinics at Stanford University Cancer Center in Palo Alto, California. |
|

