You are here: Home: CCU 4 | 2004: Jean-Yves Douillard, MD, PhD
| Jean-Yves Douillard, MD, PhD |
EDITED COMMENTS |
 Role of infusional 5-FU, bolus 5-FU and capecitabine Role of infusional 5-FU, bolus 5-FU and capecitabine
We are convinced that infusional 5-FU is more efficacious and better tolerated than bolus 5-FU. Physicians in the United States are slowly moving toward infusional 5-FU. With the FDA’s approval of oxaliplatin and the FOLFOX regimen, United States physicians will use infusional 5-FU more, but they’re still concerned about lines and pumps.
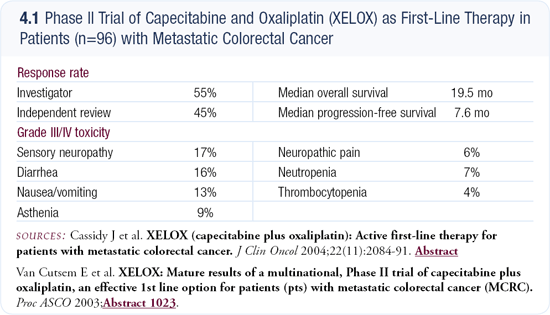
Based on the results we now have from Phase II and Phase III trials, I’m convinced capecitabine will eventually be able to replace 5-FU. For the combination regimens of capecitabine with oxaliplatin or irinotecan, we have to wait for ongoing trials to mature. In France we are conducting a trial comparing XELOX (capecitabine and oxaliplatin) to FOLFOX. The preliminary results from Europe (4.1) demonstrate a 45 to 50 percent response rate for XELOX, which is similar to the response rate for FOLFOX. If the randomized trial were to show noninferiority, we would eliminate infusional 5-FU in favor of the oral fluoropyrimidine.
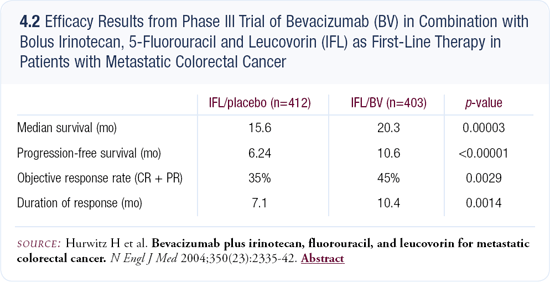
Trial evaluating IFL plus bevacizumab
The results from the trial evaluating IFL plus bevacizumab were a big surprise (4.2). It was a well-conducted study with a large number of patients, and hopefully the results will be confirmed by other trials. I believe those data are accurate, and I believe they offer a major contribution. I regret that bevacizumab was combined with IFL and not FOLFIRI (infusional 5-FU, leucovorin and irinotecan). If bevacizumab combined with FOLFIRI were to improve the survival the same percentage as when combined with IFL, median survival might be 25 to 26 months.
Implications of the MOSAIC adjuvant trial results
The MOSAIC adjuvant trial data demonstrate that combination therapy (infusional 5-FU, leucovorin and oxaliplatin) improves results compared to a single agent, which we knew was true in metastatic disease.
The trial enrolled patients with Stage II and III disease, but I would have preferred separate trials for patients with Stage II and Stage III disease. Clearly, combination therapy improved the three-year disease-free survival with a 23 percent reduction in the risk of relapse.
Generally, a disease-free survival advantage equates to an advantage in five-year survival, but I would still like to see the five-year survival data. Even though oxaliplatin is not approved as adjuvant therapy in France, I offer combination therapy with FOLFOX to young patients with a high risk of recurrence who are not enrolled in a clinical trial.
Next year we should have results from a study being conducted in France that compares FOLFIRI to single-agent 5-FU in patients at high risk with more than four positive lymph nodes. If that trial turns out to be positive, two trials will have shown that combination therapy does better than single-agent 5-FU, and we should move to combination adjuvant therapy.
Select Publications
 |
| Dr Douillard is a Professor and Head of the Department of Medical Oncology at the Centre R Gauducheau in Saint Herblain, France. |
|

