You are here: Home: CCU 3 |2004: Roy E Smith, MD, MS
| Roy E Smith, MD, MS |
EDITED COMMENTS |
 MOSAIC adjuvant trial: Implications for clinical practice MOSAIC adjuvant trial: Implications for clinical practice
The MOSAIC trial certainly had an impact on clinical practice. Ultimately, at least for the short-term, the paradigm of adjuvant therapy will switch to infusional 5-FU therapy. The data has just been published and already members of the Intergroup have decided to largely abandon FOLFOX4, which was used in the MOSAIC trial, in favor of a modified FOLFOX6 or even FOLFOX7 regimen to make it more practical for infusional therapy in the United States.
The implication is that if you had a noninfusional therapy that was equivalent or better, our treatment paradigm would immediately shift to oral therapy. Our future treatment approach may be the use of a CAPOX-type regimen or capecitabine plus another agent.
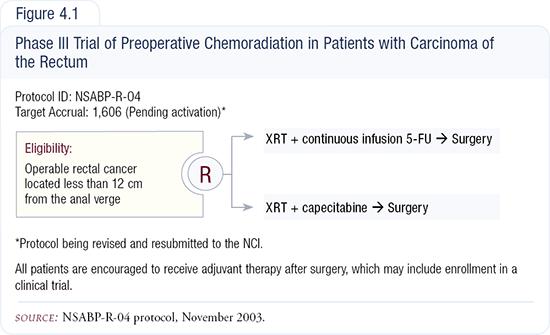
NSABP-R-04: Preoperative prolonged 5-FU venous infusion versus capecitabine during radiotherapy for resectable rectal cancer
NSABP-R-04 (Figure 4.1) was designed to be a preoperative study for patients with resectable rectal cancer because our R-03 study, which was a comparison of preoperative versus postoperative chemoradiation therapy, failed accrual as had others in the United States. There was no real equipoise among our membership in how these individuals should be treated. Approximately one-half of our surgeons felt that these patients should be treated with preoperative therapy, and one-half believed it should be postoperative. Therefore, it was unethical to enroll patients in the trial.
We convened a group of 40 to 50 rectal surgeon specialists and members of the Cancer Therapy Evaluation Program (CTEP) to decide on our next trial design. We concluded it would be far better to simply choose a preoperative trial or a postoperative trial. At that time, we were approached by a large group of rectal surgeons who have formed a nonfunded cooperative group in the United States, and they asked us to help design and implement a rectal trial but they preferred that it be preoperative.
Our rectal surgeon specialists also preferred a design that would guarantee patient compliance as much as possible, so we considered incorporating capecitabine. The evidence that radiation therapy upregulated thymidine phosphorylase levels the target enzyme for the activation of conversion of capecitabine to 5-FU suggested a reasonable possibility for synergism between capecitabine and radiation therapy, so we chose to compare capecitabine and radiation therapy versus infusional 5-FU and radiation therapy.
NSABP-C-09: CAPOX with or without intrahepatic floxuridine infusion after resection or ablation of liver metastases
NSABP-C-09 is designed so that patients with more than six individual hepatic metastases will not be eligible. Eligible patients will be treated by either surgery or ablation or a combination of the two, with quality control parameters to ensure that those procedures adequately remove the tumor burden from the liver.
Patients are randomly assigned to CAPOX or CAPOX plus hepatic arterial infusion floxuridine. During the first four cycles, the floxuridine and the capecitabine are staggered so that the patients do not receive it concurrently. The rationale for using capecitabine is that there is not only increased thymidine phosphorylase activity in the tumor but also in the liver.
Theoretically, the oral delivery of capecitabine may have the same effect as delivering intrahepatic chemotherapy because conversion of capecitabine to 5-FU results in increased concentrations of 5-FU in both tumor tissue and normal liver tissue.
We felt if we administered the floxuridine and the capecitabine simultaneously we might encounter significant toxicity and liver function problems. So, we decided to stagger their administration. After the floxuridine is administered, the two arms equilibrate in terms of their design and how the capecitabine and oxaliplatin are administered. The purpose of the study is to determine whether intrahepatic infusion is really necessary in that setting.
Select Publications
 |
| Dr Smith is CEO and President of the Regional Cancer Center in Erie, Pennsylvania, Professor of Hematology/Oncology at Drexel University, and Director of Medical Affairs for National Surgical Adjuvant and Breast Project in Erie, Pennsylvania. |
|

