You are here: Home: CCU 3 |2004: Bruce D Minsky, MD
| Bruce D Minsky, MD |
EDITED COMMENTS |
 German trial of preoperative chemoradiation in rectal cancer German trial of preoperative chemoradiation in rectal cancer
A German trial presented at the 2003 American Society for Therapeutic Radiology Oncology meeting evaluated preoperative versus postoperative chemoradiation in rectal cancer.
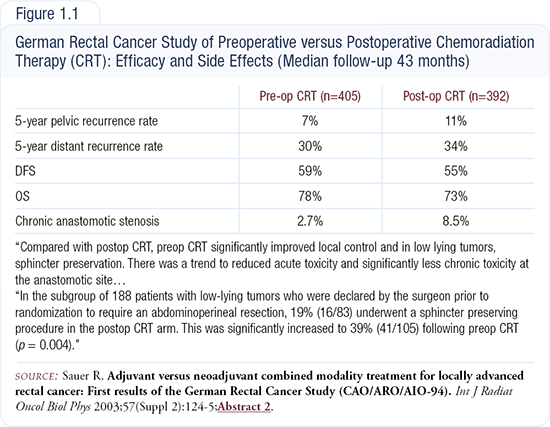
This landmark study in patients with ultrasound-confirmed Stage T3-T4 or node-positive rectal cancer was unique in that it was actually completed. Two prior studies an — Intergroup trial and NSABP-R-03 — failed because of lack of accrual. Importantly, all patients underwent a total mesorectal excision, which is now considered the standard of care for surgery. Additionally, all patients were stratified by surgeon, which is important because surgical bias exists in terms of sphincter preservation. In stratifying by surgeon, that bias is essentially removed.
The overall results of the study (Figure 1.1) are positive in that local recurrence significantly decreased from 11 percent to seven percent with preoperative therapy. Second, there was a significant decrease in both short- and long-term toxicity. Third, and most important, preoperative therapy resulted in a doubling of sphincter preservation rate from approximately 20 percent to 40 percent.
As predicted, disease-free and overall survival did not change. Another interesting finding was that there was no difference in the incidence of distant metastatic disease, which suggests that if a patient needs chemoradiation, the ideal time to deliver it is preoperatively. However, we clearly need to develop better systemic therapies to integrate into preoperative chemoradiation, as we have done for patients with advanced disease. The replacement study of the German trial will use the current preoperative arm, which is a continuous infusion of 5-FU plus preoperative radiation therapy, and compare that with capecitabine plus oxaliplatin (CAPOX), with radiation as a somewhat more intensive preoperative regimen.
Incorporation of capecitabine with preoperative radiation therapy
Emerging data support combining capecitabine and oxaliplatin with radiation therapy. The most interesting data comes from the German group led by Claus Rodel, who published his experience in the August 2003 issue of the Journal of Clinical Oncology. He performed a well-designed Phase I/II study evaluating the doses of capecitabine and oxaliplatin appropriate to combine with radiation therapy.
NSABP-R-04 is a new preoperative study in which the experimental arm is capecitabine plus radiation therapy versus the standard arm, which is continuous infusion 5-FU with radiation therapy. Initially, there was going to be a secondary randomization to erythropoietin or no erythropoietin, but that has been dropped. The study is now going forward, although it has been in negotiation with the Cancer Therapy Evaluation Program (CTEP) for approximately two years. There is still some debate because many oncologists have already adopted the experimental arm, capecitabine, as one of their treatment programs.
I want to emphasize that capecitabine is not yet the standard of care. Continuous infusion 5-FU remains the standard of care, and this would be the first study in which capecitabine is directly compared to the continuous infusion. In all of the other randomized studies in both metastatic and adjuvant colon cancer, capecitabine was compared to bolus 5-FU. This would be a valuable study, but I'm not sure if people want to wait five or seven years for the answer.
Adjuvant capecitabine versus continuous infusion 5-FU
I'm not certain capecitabine will be more efficacious than continuous infusion 5-FU, but laboratory data suggest that capecitabine is a radiosensitizer, and radiation therapy increases the activity of capecitabine by upregulating thymidine phosphorylase (TP).
There are many reasons to combine capecitabine and radiation therapy. Many of the new Phase I and II studies in the United States and Europe are using capecitabine in combination with newer agents. For example, a new RTOG study will be opening over the course of the next few months in which patients will be randomly assigned to preoperative capecitabine/oxaliplatin and radiation therapy versus irinotecan/capecitabine and radiation therapy. It is clear where development is going, at least in the investigational approach, and I suspect that's how we will treat colorectal cancer over the next year or two.
The primary motivation for using capecitabine will probably be one of convenience. Many people ask if we are ready to use capecitabine with radiation therapy today, and my answer is "not quite yet." I'm waiting to see the results of the X-ACT study, which is an adjuvant study of colon cancer patients comparing the Mayo Clinic regimen to capecitabine. It is designed to determine whether capecitabine and 5-FU are equivalent. We hope to see results reported at ASCO in 2004. If that study demonstrates equivalence, then I would feel comfortable substituting capecitabine for 5-FU.
Select Publications
 |
| Dr Minsky is Vice Chairman of the Department of Radiation Oncology at the Memorial Kettering Cancer Center and Professor of Radiation Oncology in Medicine at the Weill College of Cornell University in New York, New York. |
|

