You are here: Home: CCU 3 |2004: Bruce J Giantonio, MD
| Bruce J Giantonio, MD |
EDITED COMMENTS |
 MOSAIC trial MOSAIC trial
An impressive five-percent improvement in disease-free survival was seen with the addition of oxaliplatin in the MOSAIC trial. We have been using 5-FU/leucovorin for a decade, and I believe this is the first promising data indicating that there’s an agent that can improve efficacy without compromise.
The concern with oxaliplatin is neurotoxicity, although in my experience, this toxicity isn’t as troubling as anticipated. In the adjuvant setting, the intent is cure, so some degree of neurotoxicity may be acceptable. In advanced disease, patients who perceive a clinical benefit from the drug may be willing to tolerate some side effects. However, not all patients feel this way. It’s not clear whether agents that might prevent or ameliorate neurotoxicity, such as glutathione, calcium and magnesium, interfere with the effectiveness of oxaliplatin. These must be used cautiously and further studied.
Capecitabine in the metastatic and adjuvant settings
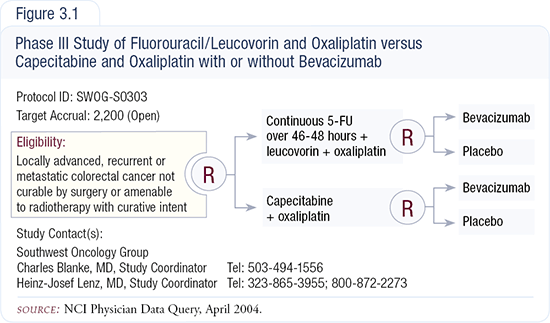
SWOG-S0303 is a Phase III trial with a two-by-two factional design, comparing FOLFOX versus CAPOX — both regimens with or without bevacizumab — in patients with advanced colorectal cancer (Figure 3.1). Off protocol, in front-line therapy, I currently use FOLFOX4 because the data is strongest for that regimen, or FOLFOX6, which is easier on patients.
The data demonstrate that capecitabine is active, but we don’t know how it compares with other regimens, and a myriad of regimens are now available. Capecitabine is an interesting drug with several advantages. Many patients prefer an oral agent. In rural communities, infusional therapies may not be an option. In the nonprotocol setting, I believe capecitabine is a reasonable choice for front-line therapy, and this trial will determine whether it is equivalent to FOLFOX.
I don’t believe capecitabine/oxaliplatin in the adjuvant setting is advisable at this time. The data is not conclusive and because the intent is cure, one should be cautious about veering away from proven therapies.
Mechanism of action of angiogenesis inhibitors
In a study presented by Herb Hurwitz at ASCO, adding bevacizumab to IFL proved to be clinically beneficial. It also proved that Dr Folkman's angiogenesis hypothesis, at least in this disease, is very likely to be true, although there are several theories as to how angiogenesis inhibitors interface with chemotherapy. One hypothesis is that tumors have a very disorganized vasculature and bevacizumab helps to stabilize that, which enhances the penetration of chemotherapeutic agents into the tumor. Bevacizumab clearly interrupts the signaling with VEGF and its receptor. I believe the long-term effect is disruption of the tumor's ability to develop its own blood supply, but there's probably also some disruption of maintenance or autocrine signaling that has an immediate effect.
Bevacizumab-related hypertension and bleeding
Our data shows a relationship between bevacizumab and hypertension and low-grade bleeding, which is mirrored by Dr Hurwitz's data. The small increase in Grade III and IV hypertension may be a result of the drug's impact on the vasculature and the glomerulus in the kidney. Management depends on the patient, the grade and whether they are on an antihypertensive drug. For Grade III or IV, I don't recommend using bevacizumab until it's resolved to at least Grade I. For Grade I hypertension, I add hydrochlorothiazide, assuming there's no contraindication, and if they are already on hydrochlorothiazide or a similar agent, I try adjusting the dose to bring it under control.
In ECOG-2200, the Phase II study of IFL/bevacizumab, approximately 38 percent of the patients had some degree of bleeding, but the vast majority of those events were Grade I and mostly epistaxis. ECOG-3200 excluded patients who required therapeutic anticoagulation, and we did not see much difference between the arms in the rates of Grade III or IV bleeding, which was Dr Hurwitz's experience as well. I believe we still need to monitor these patients, but right now we are not seeing a bleeding rate that suggests it's a significant concern.
Combination of IFL and bevacizumab in Phase II and III trials
When we look at the data from our Phase II study of IFL/bevacizumab, ECOG- 2200, with the caveat that it’s not legitimate to compare Phase II to Phase III trials, our numbers are comparable to Hurwitz’s. In ECOG-2200 the primary endpoint was improvement in progression-free survival. The reported norm for IFL is seven months, but the median progression-free survival in patients who also took bevacizumab is 10 months. Currently, the response rate is approximately 49 percent and although we have not yet reached the survival endpoint, the one-year survival rate is 84 percent.
ECOG-3201: Comparison of three chemotherapy regimens in the treatment of rectal cancer
ECOG-3201 is a Phase III adjuvant study in which patients are randomly assigned to three different chemotherapy regimens — irinotecan/5-FU/leucovorin versus oxaliplatin/5-FU/leucovorin versus 5-FU/leucovorin — for the management of Stage II or III rectal cancer. A unique aspect of this study is that the treating physician (rather than the protocol) decides whether the patient receives chemoradiation before or after surgery, which also affects randomization. This gives the physician greater flexibility, which should improve accrual. We’ve learned to design studies that are more user-friendly while maintaining scientific integrity.
Select Publications
 |
| Dr Giantonio is an Assistant Professor of Medicine at the Abramson Cancer Center of the University of Pennsylvania in Philadelphia, Pennsylvania. |
|

