You are here: Home: CCU 3 |2004: Nancy E Kemeny, MD
| Nancy E Kemeny, MD |
EDITED COMMENTS |
 Addition of dexamethasone and leucovorin to hepatic arterial infusion (HAI) FUDR Addition of dexamethasone and leucovorin to hepatic arterial infusion (HAI) FUDR
FUDR is the drug that we used for the hepatic infusion during our initial trials. You don't see diarrhea, vomiting and hair loss with this type of therapy. The major side effect is hepatic toxicity and the most serious toxicity occurs when the bile ducts are damaged.
The hepatic artery feeds the bile ducts just like it does the tumor, therefore profusing the artery and getting rid of the tumor will also affect the ducts.
We thought that this damage was an inflammation so we decided to add dexamethasone. By doing so, we were able decrease the number of patients with elevated bilirubins, but even more interestingly we also increased the response rate and the survival compared to FUDR alone. We couldn't understand why this happened, but when we went back to the laboratory we found out that dexamethasone actually increases the cytotoxicity of FUDR.
We also experimented by adding leucovorin to our regimen because in systemic trials, the addition of leucovorin increased response rates of 5-FU. We observed responses as high as 73 percent in patients with metastatic disease, and those were the days when the response rate with 5-FU/leucovorin was about 20 percent.
Intergroup trial comparing HAI versus systemic therapy
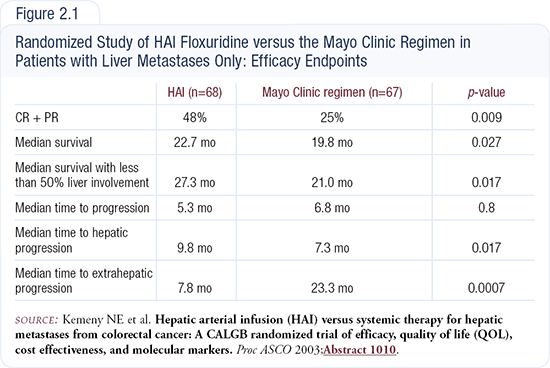
Last year at ASCO we presented the results of a large Intergroup trial comparing 5-FU/leucovorin, which was the best combination at that time, to hepatic arterial infusion (Figure 2.1). The study took years to complete and accrual was very slow for several reasons.
Patients who want hepatic therapy do not want to be randomly assigned to receive systemic therapy, and at the time, some institutions believed in hepatic therapy while others believed in systemic therapy. Another problem with the study was that there was no crossover.
Patients with less than 70 percent liver-only involvement who were not previously treated with systemic therapy — except for adjuvant therapy completed 12 months before entry — were eligible for the study. We accrued 140 patients, of whom 79 percent entered the trial with synchronous disease. Presentation with liver metastases is an adverse prognostic variable, and those patients usually do much worse in terms of survival.
Median survival was 22.7 months for the hepatic group and 19.8 months for the systemic group. The response rate was 51 percent for the hepatic group and 24 percent for the systemic group. In patients with less than 50 percent liver involvement, the median survival was 27 months with hepatic arterial treatment. Patients with more than 50 percent liver involvement based on CAT scan usually have a very poor prognosis.
MSKCC trial of HAI plus systemic therapy after hepatic resection
We know that surgical removal of the tumor results in better survival. However, we also know that about 70 percent of patients have a recurrence after surgery, and about half of those recurrences are confined to the liver.
At Memorial, we decided to add hepatic arterial therapy plus systemic therapy after resection to give maximum treatment. We felt if we could treat aggressively, both in the liver and systemically, we could potentially improve survival and decrease the rate of recurrence.
We designed a randomized study comparing hepatic arterial therapy with floxuridine and dexamethasone plus systemic therapy with 5-FU/leucovorin versus systemic 5-FU/leucovorin alone. We chose not to have a complete control arm because we didn't think we'd get accrual, so one group received combined treatment and one group received systemic therapy alone.
The usual two-year survival for nonresectable metastatic disease is 20 percent; the five-year survival rate for resectable disease without any treatment is 30 percent. In this trial, the two-year survival rate was 86 percent for the combined treatment arm and 62 percent for the systemic therapy alone arm. At five years, the survival rates were 56 percent versus 45 percent, respectively. We now have 10-year data that indicate a survival rate of 40 percent in patients who received combined therapy versus 20 percent in patients who received systemic therapy alone. The p-value for the entire curve is still not significant, but we only had 156 patients.
The patients who were alive at 10 years were mostly free of disease. We saw some recurrences in both arms of the study; however, 70 percent of patients in the combined therapy group have not had a liver recurrence compared to only 40 percent in patients who received systemic therapy alone. That is a very significant difference even though the study wasn't powered to evaluate that.
NSABP-C-09: Capecitabine/oxaliplatin (CAPOX) versus CAPOX plus HAI floxuridine
The NSABP has designed a proposed study comparing oxaliplatin/capecitabine and oxaliplatin/capecitabine in combination with the pump. I was somewhat surprised that they initiated this study without even looking at our data. We have found that when you use oxaliplatin with the pump, it's harder to get the pump treatment in. Oxaliplatin must affect the liver more than irinotecan because with oxaliplatin you have to reduce the floxuridine dose a bit and it causes more toxicity. We know that in patients receiving oxaliplatin before surgery, a lot of fatty changes are observed during the procedure. It may be that the drug is affecting the liver more than we think.
I believe pilot studies should be done before adjuvant studies, and you should have more data before you subject a lot of patients to a randomized study. The NSABP went very quickly into the adjuvant arena without a real pilot study.
Select Publications
 |
| Dr Kemeny is an Attending Physician at the Memorial Sloan-Kettering Cancer Center and of Medicine at the Weill Medical College of Cornell University in New York, New York |
|

