
 |
|||||||

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Discussion
Tracks 1, 4-5, 8

![]() DR LOVE: What treatment options would you consider for this patient,
outside of a clinical trial?
DR LOVE: What treatment options would you consider for this patient,
outside of a clinical trial?
![]() DR MEROPOL: The first treatment option would be FOLFOX in combination
with bevacizumab — considering she did not experience a tremendous
amount of toxicity from FOLFOX, and she had a two-year disease-free
interval. Another treatment option would be the combination of irinotecan,
5-FU and bevacizumab.
DR MEROPOL: The first treatment option would be FOLFOX in combination
with bevacizumab — considering she did not experience a tremendous
amount of toxicity from FOLFOX, and she had a two-year disease-free
interval. Another treatment option would be the combination of irinotecan,
5-FU and bevacizumab.
I would consider those to be the two standard treatment options. She had not received irinotecan or an antibody against the epidermal growth factor receptor (EGFR).
Those were the drugs on the table, but in taking a sequential approach, I thought it made the most sense to offer chemotherapy in combination with bevacizumab.
![]() DR LOVE: Alan, it sounds as though this patient might be eligible for your
study, CALGB-C80405, evaluating the combination of chemotherapy and
biologic agents as front-line therapy for metastatic colorectal cancer. Can you
describe the design of that trial?
DR LOVE: Alan, it sounds as though this patient might be eligible for your
study, CALGB-C80405, evaluating the combination of chemotherapy and
biologic agents as front-line therapy for metastatic colorectal cancer. Can you
describe the design of that trial?
![]() DR VENOOK: In this trial, the choice of chemotherapy regimen — FOLFOX
or FOLFIRI — is left up to the physician and the patient to decide. Then
patients are randomly assigned to cetuximab alone, cetuximab with bevacizumab
or bevacizumab alone (1.1). Indeed, a patient such as this one — who
completed adjuvant FOLFOX more than a year earlier — would be eligible
for enrollment.
DR VENOOK: In this trial, the choice of chemotherapy regimen — FOLFOX
or FOLFIRI — is left up to the physician and the patient to decide. Then
patients are randomly assigned to cetuximab alone, cetuximab with bevacizumab
or bevacizumab alone (1.1). Indeed, a patient such as this one — who
completed adjuvant FOLFOX more than a year earlier — would be eligible
for enrollment.
![]() DR LOVE: Axel, in terms of the issue of combining bevacizumab with an
anti-EGFR antibody, what exactly do we know about the PACCE trial and
panitumumab?
DR LOVE: Axel, in terms of the issue of combining bevacizumab with an
anti-EGFR antibody, what exactly do we know about the PACCE trial and
panitumumab?
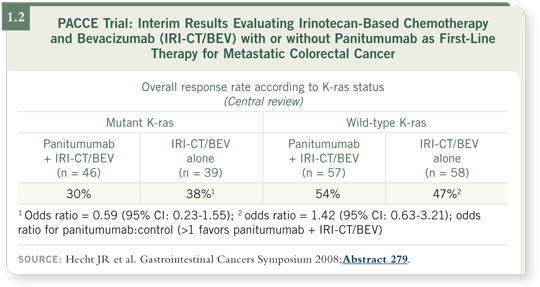
![]() DR GROTHEY: The PACCE trial had two cohorts — one cohort received an
oxaliplatin-based regimen, and the other cohort received an irinotecan-based
regimen. The data from the cohort that received irinotecan-based chemotherapy
were reported. Approximately 200 patients were randomly assigned
to irinotecan/5-FU/bevacizumab with or without panitumumab as first-line
therapy.
DR GROTHEY: The PACCE trial had two cohorts — one cohort received an
oxaliplatin-based regimen, and the other cohort received an irinotecan-based
regimen. The data from the cohort that received irinotecan-based chemotherapy
were reported. Approximately 200 patients were randomly assigned
to irinotecan/5-FU/bevacizumab with or without panitumumab as first-line
therapy.
The data revealed the activity of panitumumab — an antibody that targets EGFR — in patients with tumors that had wild-type versus mutant K-ras. Patients whose tumors had mutant K-ras received no benefit — in terms of response rate — from panitumumab in combination with irinotecan/5-FU/bevacizumab.
In contrast, patients whose tumors had wild-type K-ras had a higher response rate when panitumumab was combined with irinotecan/5-FU/bevacizumab (Hecht 2008; [1.2]).
![]() DR LOVE: What proportion of patients have a tumor with a K-ras mutation?
DR LOVE: What proportion of patients have a tumor with a K-ras mutation?
![]() DR GROTHEY: Approximately 40 to 45 percent have a K-ras mutation. One
caveat is that those patients have a poorer prognosis.
DR GROTHEY: Approximately 40 to 45 percent have a K-ras mutation. One
caveat is that those patients have a poorer prognosis.

Tracks 11, 13, 15

![]() DR LOVE: Alan, does this woman have high-risk Stage II disease?
DR LOVE: Alan, does this woman have high-risk Stage II disease?
![]() DR VENOOK: Her high-risk feature was the 11 lymph nodes examined. She
had radiographic evidence of a near obstruction, but she was asymptomatic.
After a long discussion with the patient and her family, we decided not to use
adjuvant therapy.
DR VENOOK: Her high-risk feature was the 11 lymph nodes examined. She
had radiographic evidence of a near obstruction, but she was asymptomatic.
After a long discussion with the patient and her family, we decided not to use
adjuvant therapy.
![]() DR MEROPOL: The fact that she had no symptoms is important in assessing
her risk. The studies that examined obstruction as an indicator of poor
prognosis or a high-risk feature were conducted in patients who presented
with frank bowel obstruction or clear radiographic evidence of a bowel
obstruction that required emergency surgery.
DR MEROPOL: The fact that she had no symptoms is important in assessing
her risk. The studies that examined obstruction as an indicator of poor
prognosis or a high-risk feature were conducted in patients who presented
with frank bowel obstruction or clear radiographic evidence of a bowel
obstruction that required emergency surgery.
![]() DR LOVE: What about the number of nodes examined? Interestingly, the
Adjuvant! Online model uses a cutoff of 10 nodes.
DR LOVE: What about the number of nodes examined? Interestingly, the
Adjuvant! Online model uses a cutoff of 10 nodes.
![]() DR MEROPOL: A number of studies suggest that the number of lymph nodes
evaluated is probably a continuous variable. For quality assurance purposes, the
notion was accepted that the evaluation of 12 lymph nodes was a good cutoff
and the number we should aspire to and that patients with fewer than 12 nodes
examined ought to be considered at high risk, at least in some way.
DR MEROPOL: A number of studies suggest that the number of lymph nodes
evaluated is probably a continuous variable. For quality assurance purposes, the
notion was accepted that the evaluation of 12 lymph nodes was a good cutoff
and the number we should aspire to and that patients with fewer than 12 nodes
examined ought to be considered at high risk, at least in some way.
I have two comments regarding this issue. First, in general, it’s not dichotomous — fewer than 12, more than 12. Indeed, 11 lymph nodes is pretty close to 12, and it is not the same situation as a patient who has zero nodes evaluated. Second, a contrarian view was raised in a paper published in the Journal of the American Medical Association using a large administrative data set, which suggested that in Stage II colon cancer, the number of lymph nodes examined does not imply risk (Wong 2007).
![]() DR LOVE: Neal, this woman is 80 years old. How would each of you treat an
otherwise healthy, 55-year-old patient who has Stage II disease without any
high-risk factors?
DR LOVE: Neal, this woman is 80 years old. How would each of you treat an
otherwise healthy, 55-year-old patient who has Stage II disease without any
high-risk factors?
![]() DR MEROPOL: For me, it’s probably a 50/50 split between treating with a
fluoropyrimidine — usually capecitabine — or nothing.
DR MEROPOL: For me, it’s probably a 50/50 split between treating with a
fluoropyrimidine — usually capecitabine — or nothing.
![]() DR VENOOK: I agree. I would use capecitabine alone or nothing at all,
depending on other issues.
DR VENOOK: I agree. I would use capecitabine alone or nothing at all,
depending on other issues.
![]() DR GROTHEY: I find it useful to sit down with patients, view their case on
Adjuvant! Online and print out the data. I would not exclude oxaliplatin-based
therapy for these patients.
DR GROTHEY: I find it useful to sit down with patients, view their case on
Adjuvant! Online and print out the data. I would not exclude oxaliplatin-based
therapy for these patients.
Tracks 16-17

![]() DR LOVE: How did you approach this patient, Axel?
DR LOVE: How did you approach this patient, Axel?
![]() DR GROTHEY: My first discussion with the patient included asking what he
wanted to accomplish with therapy. He was recently widowed, was depressed
and lived alone.
DR GROTHEY: My first discussion with the patient included asking what he
wanted to accomplish with therapy. He was recently widowed, was depressed
and lived alone.
At first he said, “I don’t know whether it’s worth it.” Then he asked, “So, what are we talking about here?” I explained that considering how his tumor was progressing and his physical condition, if we did not use antitumor therapy, he might survive only a matter of weeks, but I believed if the tumor responded well to therapy, being alive in a year was achievable and I would consider that a success.
He decided he wanted therapy, and we started with FOLFOX. I did not administer bevacizumab during the first cycle. I didn’t even implant a Port-A-Cath® because I wasn’t certain we’d complete the first cycle of FOLFOX. I saw him weekly because I wanted to make sure he didn’t develop any problems, and I omitted the bolus 5-FU to reduce the risk of complications.
The bleeding stopped almost immediately, his bilirubin dropped significantly and he felt better. I added bevacizumab from the second cycle on. After four cycles of therapy, his liver was no longer palpable. Initially, his CEA level was in the range of 500 ng/mL, and it normalized with therapy, as did his LDH level. It was one of the most dramatic responses I have ever seen.
Although he benefited from therapy, it didn’t last long. He had problems with toxicities in the end. In spite of omitting the bolus 5-FU, he had some neutropenia and infectious complications. His tumor was controlled as long as we continued therapy, but about nine months later he said, “I think this is it. We’ve done exactly what we wanted to do, and you’ve given me some time.” So we stopped therapy, and he died almost exactly one year after he started therapy.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |