
 |
|||||||

| Tracks 1-13 | ||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: Can you discuss the analysis of CALGB-C89803 with regard
to the influence of diet and exercise on colon cancer recurrence?
DR LOVE: Can you discuss the analysis of CALGB-C89803 with regard
to the influence of diet and exercise on colon cancer recurrence?
![]() DR MAYER: Jeff Meyerhardt and Charlie Fuchs, based on their experience
with the Nurses’ Health Study, developed a prospective questionnaire about the
effects of diet, exercise and lifestyle on colon cancer recurrence. We have also
collected blood samples, and we’ll be able to determine prospectively if different
factors, such as cytokines and insulin growth factor receptors, correlate.
DR MAYER: Jeff Meyerhardt and Charlie Fuchs, based on their experience
with the Nurses’ Health Study, developed a prospective questionnaire about the
effects of diet, exercise and lifestyle on colon cancer recurrence. We have also
collected blood samples, and we’ll be able to determine prospectively if different
factors, such as cytokines and insulin growth factor receptors, correlate.
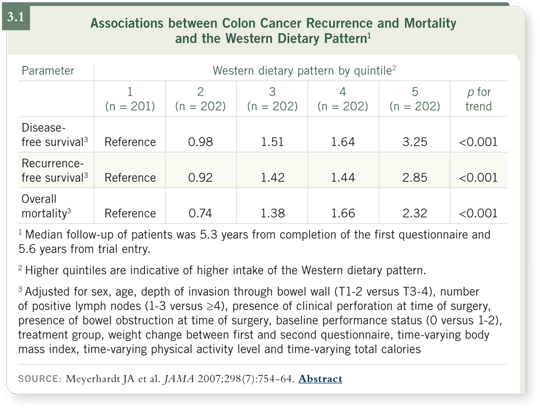
They found that a Western diet that includes lots of fats and obesity may be associated with a higher risk of recurrence and mortality (Meyerhardt 2007; [3.1]). Perhaps, as you become obese or develop type II diabetes, you also stimulate a variety of hormone cytokines — factors that may activate microscopic tumor cells. A strong association between exercise and reduced risk of cancer relapse was also seen (Meyerhardt 2006).
Track 4
![]() DR LOVE: What are your thoughts on evolving data evaluating K-ras and
the EGFR inhibitors?
DR LOVE: What are your thoughts on evolving data evaluating K-ras and
the EGFR inhibitors?
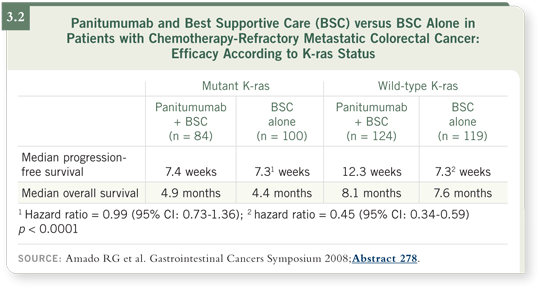
![]() DR MAYER: A paper recently published in the Journal of Clinical Oncology had
fascinating, clearly stated data on the correlation of K-ras mutations and lack of
response to cetuximab (Lièvre 2008). At the ASCO GI 2008 meeting, Amado
and colleagues presented panitumumab data from Europe broken down by
K-ras mutation status (Amado 2008; [3.2]). These data explain to an enormous
degree why the combination regimen in the large Phase III SWOG-S0205 trial
evaluating gemcitabine/cetuximab versus gemcitabine alone in patients with
pancreatic cancer showed no benefit (Phillip 2007), because essentially 98 to
99 percent of pancreatic cancer cases have K-ras mutations — those tumors do
not respond to treatment. In colon cancer, approximately 40 percent of patients
have the K-ras mutation.
DR MAYER: A paper recently published in the Journal of Clinical Oncology had
fascinating, clearly stated data on the correlation of K-ras mutations and lack of
response to cetuximab (Lièvre 2008). At the ASCO GI 2008 meeting, Amado
and colleagues presented panitumumab data from Europe broken down by
K-ras mutation status (Amado 2008; [3.2]). These data explain to an enormous
degree why the combination regimen in the large Phase III SWOG-S0205 trial
evaluating gemcitabine/cetuximab versus gemcitabine alone in patients with
pancreatic cancer showed no benefit (Phillip 2007), because essentially 98 to
99 percent of pancreatic cancer cases have K-ras mutations — those tumors do
not respond to treatment. In colon cancer, approximately 40 percent of patients
have the K-ras mutation.
Track 6
![]() DR LOVE: Where do you think we might be headed in terms of the
concept of double antibody therapy for advanced colorectal cancer?
DR LOVE: Where do you think we might be headed in terms of the
concept of double antibody therapy for advanced colorectal cancer?
![]() DR MAYER: That’s a controversial issue because of the results of the PACCE
study presented at the 2008 ASCO GI meeting (Hecht 2008a, 2008b). The
PACCE study is a randomized trial in which four out of five patients receive FOLFOX — the remaining patients receive FOLFIRI — and are then randomly
assigned to receive bevacizumab alone or with panitumumab. To everyone’s
surprise, PACCE has shown seeming detriment and increased toxicity.
DR MAYER: That’s a controversial issue because of the results of the PACCE
study presented at the 2008 ASCO GI meeting (Hecht 2008a, 2008b). The
PACCE study is a randomized trial in which four out of five patients receive FOLFOX — the remaining patients receive FOLFIRI — and are then randomly
assigned to receive bevacizumab alone or with panitumumab. To everyone’s
surprise, PACCE has shown seeming detriment and increased toxicity.
Some people argue that bevacizumab works as an anti-angiogenesis drug. However, others would argue that bevacizumab works by increasing the permeability of the cell membranes, thereby modulating chemotherapy and increasing chemotherapy concentrations within the cell. Could that be interfering with the binding of a compound such as cetuximab or panitumumab to the cell surface? We do not have answers at the moment, and the analysis isn’t yet in on the PACCE study.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |