
 |
|||||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2, 4
![]() DR LOVE: Can you discuss the results from the EORTC-40983 trial?
DR LOVE: Can you discuss the results from the EORTC-40983 trial?
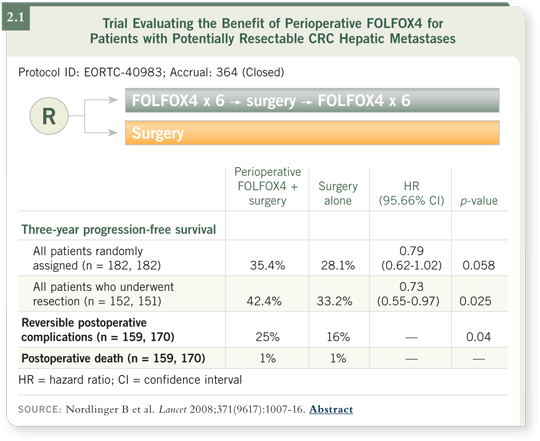
![]() PROF VAN CUTSEM: In the EORTC-40983 study, patients with resectable
liver metastases received six cycles of FOLFOX4 before and after resection
(perioperative chemotherapy). The progression-free survival for the patients
treated with perioperative chemotherapy was better than that for patients who underwent surgery alone. We also saw slightly more postoperative complications
among the patients who received perioperative chemotherapy than among
those who underwent surgery alone. Although slightly more morbidity was
observed, the postoperative mortality was identical (Nordlinger 2008; [2.1]).
PROF VAN CUTSEM: In the EORTC-40983 study, patients with resectable
liver metastases received six cycles of FOLFOX4 before and after resection
(perioperative chemotherapy). The progression-free survival for the patients
treated with perioperative chemotherapy was better than that for patients who underwent surgery alone. We also saw slightly more postoperative complications
among the patients who received perioperative chemotherapy than among
those who underwent surgery alone. Although slightly more morbidity was
observed, the postoperative mortality was identical (Nordlinger 2008; [2.1]).
The three-month duration of preoperative treatment is important. In other studies, when patients received more than three months of treatment, the complication rate increased. Patients with initially unresectable metastases should undergo surgery as soon as their disease becomes resectable. If the patient’s disease becomes resectable, chemotherapy should not continue until a maximum response is observed.
One reason for this is the increased risk of complications. The other reason is that if you continue to treat, the metastases may cease to appear on a CT scan, which can be a nightmare for the surgeons. Perhaps that’s an overstatement, but it’s extremely difficult for them to find the lesions if they don’t see the correlation on imaging. Upon resection, more than 80 percent of the areas in which there was initially a metastasis but then nothing is seen on a CT scan will still have microscopic lesions.
![]() DR LOVE: Could we have achieved the same results with postoperative
therapy alone?
DR LOVE: Could we have achieved the same results with postoperative
therapy alone?
![]() PROF VAN CUTSEM: Formally, we do not have proof that perioperative
followed by postoperative therapy is better than only postoperative therapy.
However, for rectal cancer and many other types of cancer, preoperative
treatment has several advantages: It’s better tolerated, and there are oncologic
advantages. At ASCO last year, Nick Petrelli suggested a randomized trial
to evaluate these two options. I believe it would be extremely difficult to
conduct such a trial, and more important questions must be addressed to make
progress in these patients.
PROF VAN CUTSEM: Formally, we do not have proof that perioperative
followed by postoperative therapy is better than only postoperative therapy.
However, for rectal cancer and many other types of cancer, preoperative
treatment has several advantages: It’s better tolerated, and there are oncologic
advantages. At ASCO last year, Nick Petrelli suggested a randomized trial
to evaluate these two options. I believe it would be extremely difficult to
conduct such a trial, and more important questions must be addressed to make
progress in these patients.
Tracks 10-11
![]() DR LOVE: Where are we now in terms of clinical research on bevacizumab?
DR LOVE: Where are we now in terms of clinical research on bevacizumab?
![]() PROF VAN CUTSEM: We have seen in the past year — in a formal randomized
trial in the first-line setting — that bevacizumab increases the activity
of oxaliplatin-based regimens (Saltz 2008). We already knew from ECOG-E3200,
the second-line study of FOLFOX with or without bevacizumab, that
the addition of bevacizumab was positive (Giantonio 2007). We also knew
that bevacizumab increased the activity of 5-FU (Hurwitz 2005) and irinotecan/5-FU (Hurwitz 2004) as first-line therapy. So the picture is becoming
more complete, and more formal evidence is accumulating.
PROF VAN CUTSEM: We have seen in the past year — in a formal randomized
trial in the first-line setting — that bevacizumab increases the activity
of oxaliplatin-based regimens (Saltz 2008). We already knew from ECOG-E3200,
the second-line study of FOLFOX with or without bevacizumab, that
the addition of bevacizumab was positive (Giantonio 2007). We also knew
that bevacizumab increased the activity of 5-FU (Hurwitz 2005) and irinotecan/5-FU (Hurwitz 2004) as first-line therapy. So the picture is becoming
more complete, and more formal evidence is accumulating.
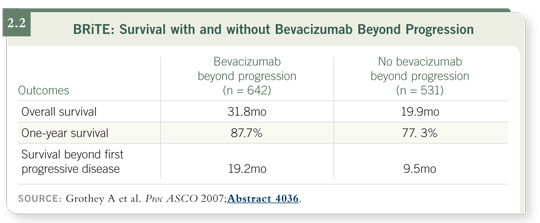
An issue we don’t have a formal answer to is the continuation of bevacizumab after disease progression. Data from the BRiTE registry — presented last year at ASCO — suggested that for patients whose disease is progressing on chemotherapy and bevacizumab, switching the chemotherapy but continuing bevacizumab produces a better outcome (Grothey 2007; [2.2]).
It’s not a randomized trial, but the BRiTE data and some preclinical data suggest that continuation of bevacizumab might benefit at least a subgroup of patients. We need the formal comparison conducted in this setting.
![]() DR LOVE: Is that what’s going to happen in the iBET study?
DR LOVE: Is that what’s going to happen in the iBET study?
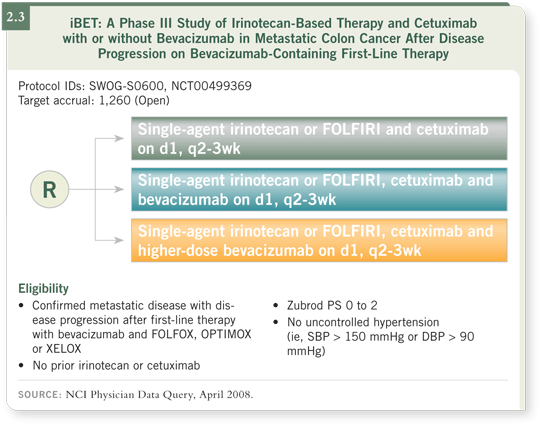
![]() PROF VAN CUTSEM: Yes. iBET is presently recruiting (2.3). However, I understand
from my American colleagues that accrual is not progressing quickly. A
German group is conducting a similar but more flexible study in that patients
can be treated with any irinotecan-or oxaliplatin-based regimen with bevacizumab
as first-line therapy, and then they change to another chemotherapy
regimen with or without bevacizumab in the second-line setting.
PROF VAN CUTSEM: Yes. iBET is presently recruiting (2.3). However, I understand
from my American colleagues that accrual is not progressing quickly. A
German group is conducting a similar but more flexible study in that patients
can be treated with any irinotecan-or oxaliplatin-based regimen with bevacizumab
as first-line therapy, and then they change to another chemotherapy
regimen with or without bevacizumab in the second-line setting.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2008 Research To Practice. All Rights Reserved. |