
| Tracks 1-15 |
| Track 1 |
Current clinical trial objectives in rectal cancer |
| Track 2 |
Clinical trials evaluating oxaliplatin as part of neoadjuvant therapy of rectal cancer |
| Track 3 |
Use of capecitabine as part of treatment of rectal cancer |
| Track 4 |
Combining EGFR inhibition with radiation therapy in rectal cancer |
| Track 5 |
Vascular normalization as a rationale for combining bevacizumab with radiation therapy |
| Track 6 |
Tumor and nodal staging errors with the use of endoscopic ultrasound |
| Track 7 |
Role of MRI in rectal cancer |
|
| Track 8 |
Treatment algorithm for local and
systemic therapy of rectal cancer |
| Track 9 |
Use of postoperative adjuvant chemotherapy for rectal cancer |
| Track 10 |
Adequacy of lymph node sampling in rectal cancer |
| Track 11 |
Treatment for patients presenting with synchronous primary rectal tumors and metastatic disease |
| Track 12 |
Abdominoperineal (AP) resection in the community versus specialty centers |
| Track 13 |
Local excision for rectal cancer |
| Track 14 |
New directions in the treatment of
anal cancer |
| Track 15 |
Modification of radiation therapy
techniques in the treatment of
anal cancer |
|
|
Select Excerpts from the Interview
Tracks 1-2
 DR LOVE:
DR LOVE: Can you provide an overview of current clinical trials in rectal
cancer?
 DR TEPPER: The major emphases in clinical trial development in rectal cancer
are in two separate areas. One is trying to enhance local control to the point of
being able to treat rectal cancer without a surgical resection. Virtually all those
trials have included radiation therapy and all include a fluoropyrimidine.
DR TEPPER: The major emphases in clinical trial development in rectal cancer
are in two separate areas. One is trying to enhance local control to the point of
being able to treat rectal cancer without a surgical resection. Virtually all those
trials have included radiation therapy and all include a fluoropyrimidine.
People are also interested in using other agents to enhance response to radiation
therapy. The new cytotoxics have been studied to a great extent. Irinotecan
has been of some interest but is problematic due to the possibility of diarrhea associated with irinotecan being additive to the diarrhea already
associated with radiation therapy and the fluoropyrimidine.
Much more interest has been shown in oxaliplatin, which has been evaluated in
Phase I and II studies. We performed a Phase I study and the initial parts of a
Phase II study, which then went into CALGB as a Phase II study (Ryan 2006).
The study’s aim was to deliver the oxaliplatin in such a way as to optimize
radiation sensitization. We used a once-weekly schedule throughout the course
of radiation therapy, which is somewhat different from the schedule typically
used with oxaliplatin alone as a cytotoxic. The Phase I study suggested that a
dose of 60 mg/m2 per week would be most appropriate.
Track 3
 DR LOVE:
DR LOVE: Have you made any observations about the potential side
effects and toxicity of capecitabine with radiation therapy as opposed to
continuous infusion 5-FU?
 DR TEPPER: After treating a number of patients, I don’t believe the side
effects to be much different. Some questions related to timing remain
regarding the combination of capecitabine with radiation therapy. Based on
some of the available pharmacokinetic data, we try to deliver the capecitabine
approximately an hour and a half before the radiation therapy. I don’t know if
that’s better, but it matches up with being at or slightly past the peak concentration
of the agent.
DR TEPPER: After treating a number of patients, I don’t believe the side
effects to be much different. Some questions related to timing remain
regarding the combination of capecitabine with radiation therapy. Based on
some of the available pharmacokinetic data, we try to deliver the capecitabine
approximately an hour and a half before the radiation therapy. I don’t know if
that’s better, but it matches up with being at or slightly past the peak concentration
of the agent.
 DR LOVE: Any reason to believe that capecitabine might be more efficacious
than 5-FU when combined with radiation therapy? There has been discussion
about whether radiation therapy increases thymidine phosphorylase (TP).
Could that in some way synergize with capecitabine better than 5-FU?
DR LOVE: Any reason to believe that capecitabine might be more efficacious
than 5-FU when combined with radiation therapy? There has been discussion
about whether radiation therapy increases thymidine phosphorylase (TP).
Could that in some way synergize with capecitabine better than 5-FU?
 DR TEPPER: Yes, the issue of the synergism has been raised, but I’m aware of
no clinical data to indicate that it is affecting the overall outcome. The response
data appear similar between the agents based on early results, but it’s possible
that the NSABP-R-04 study will demonstrate the superiority of capecitabine.
DR TEPPER: Yes, the issue of the synergism has been raised, but I’m aware of
no clinical data to indicate that it is affecting the overall outcome. The response
data appear similar between the agents based on early results, but it’s possible
that the NSABP-R-04 study will demonstrate the superiority of capecitabine.
Track 5
 DR LOVE:
DR LOVE: What are your thoughts regarding combining bevacizumab
with radiation therapy?
 DR TEPPER: Bevacizumab is an interesting drug to consider combining with
radiation therapy. You would expect that the last thing you would want to
do would be to use an anti-angiogenic agent with radiation therapy because
shutting down the blood supply could lead to worse results by producing more
hypoxic cells and a decreased response to radiation therapy.
DR TEPPER: Bevacizumab is an interesting drug to consider combining with
radiation therapy. You would expect that the last thing you would want to
do would be to use an anti-angiogenic agent with radiation therapy because
shutting down the blood supply could lead to worse results by producing more
hypoxic cells and a decreased response to radiation therapy.
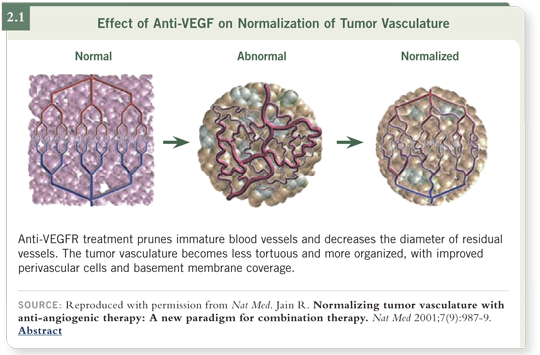
That does not appear to be the case because the preclinical data suggest that
drugs such as bevacizumab have a beneficial effect when combined with radiation therapy. Work from Rakesh Jain has suggested that what is occurring in
these tumors treated with bevacizumab is vascular normalization rather than
overall vascular shutdown ( Jain 2001; [2.1]).
By changing intratumoral pressure, we might actually allow better blood flow,
better delivery of chemotherapy and better oxygenation effects for radiation
therapy.
Few small studies have used bevacizumab with radiation therapy. Thus far, the
toxicity appears to be acceptable, but I believe it’s too early to say how encouraged
one should be with the results.
Select Publications

