
| Tracks 1-9 |
| Track 1 |
Clinical implications of the updated analyses of the MOSAIC adjuvant trial |
| Track 2 |
Adjuvant FOLFOX in elderly patients with CRC |
| Track 3 |
NCCTG adjuvant trial N0147 of FOLFOX with or without cetuximab |
| Track 4 |
Factors contributing to inadequate lymph node sampling in CRC |
| Track 5 |
Recent clinical trial results in mCRC |
|
| Track 6 |
Use of FOLFOX/bevacizumab as
first-line therapy in mCRC |
| Track 7 |
Clinical trial strategies to evaluate
novel therapies |
| Track 8 |
Evaluating agents as potential
radiation sensitizers in rectal
cancer, including capecitabine,
oxaliplatin and bevacizumab |
| Track 9 |
ACOSOG trial of laparoscopic
versus conventional resection of
rectal cancer |
|
|
Select Excerpts from the Interview
Track 1
 DR LOVE:
DR LOVE: What’s new in adjuvant therapy?
 DR GOLDBERG: The most important data regarding adjuvant therapy for
colon cancer are from the update of the MOSAIC trial (de Gramont 2007). It
hasn’t changed what we expected, but it has confirmed what we hoped. Prior
to this update, we’ve had only disease-free survival data. Now we have significant
advantage for overall survival at six years.
DR GOLDBERG: The most important data regarding adjuvant therapy for
colon cancer are from the update of the MOSAIC trial (de Gramont 2007). It
hasn’t changed what we expected, but it has confirmed what we hoped. Prior
to this update, we’ve had only disease-free survival data. Now we have significant
advantage for overall survival at six years.
The good news from MOSAIC was that the patients with Stage III disease
definitely gained approximately a five percent survival advantage. The bad
news was that the patients with Stage II disease did not appear to gain a significant
survival advantage with FOLFOX over 5-FU/leucovorin.
What does that mean? For all comers with Stage II disease, the ASCO guidelines
still apply (Benson 2004). You need to have an individualized discussion
with those patients, tell them what they can expect and ask them whether
that’s enough for them to receive adjuvant treatment. I’m always on the fence about whether to offer FOLFOX or just a fluoropyrimidine to patients with
Stage II disease who want treatment.
Track 2
 DR LOVE:
DR LOVE: How do you approach the issue of adjuvant therapy for the
older patient?
 DR GOLDBERG: I conducted a meta-analysis with Dan Sergeant of four
different trials, comparing patients older than age 70 to those younger than
age 70 (Goldberg 2006).
DR GOLDBERG: I conducted a meta-analysis with Dan Sergeant of four
different trials, comparing patients older than age 70 to those younger than
age 70 (Goldberg 2006).
We examined the MOSAIC trial, two first-line advanced-disease trials —
CALGB-9741 and the de Gramont original study — and the Rothenberg trial
in the second-line setting.
Across the board in all three settings, we found that patients benefited equally,
regardless of age. Toxicity was minimally elevated in older patients. White
blood count and thrombocytopenia were affected.
However, these were laboratory parameters, not clinical parameters. Except in
the second-line setting, the superior FOLFOX therapy conferred an advantage
of equal value to younger and older patients.
We didn’t have many patients over the age of 80, and the people enrolled were
the healthiest of the older patients. Taking the data from them and extrapolating
to the patient who walks into your office every day is more complicated.
My hope is that this study will enable oncologists to think more liberally
about the use of FOLFOX for older patients.
In my practice, if I’m worried about a patient’s ability to tolerate the regimen, I’ll
start with 5-FU/leucovorin. If they tolerate that, I’ll move up to full-dose oxaliplatin
or, if I’m being cautious, I’ll initially administer 60 mg/m2 of oxaliplatin.
If they pass that test, I’ll increase it to the full dose. I don’t believe you have to
make a decision when you first see the patient: “I will administer FOLFOX or
only 5-FU.”
 DR LOVE: What about the patient over the age of 80? Does an age limit exist
at which you will stop using adjuvant therapy?
DR LOVE: What about the patient over the age of 80? Does an age limit exist
at which you will stop using adjuvant therapy?
 DR GOLDBERG: If patients have comorbidities that suggest they aren’t likely to
live for more than two or three years, it’s reasonable not to administer adjuvant
therapy.
DR GOLDBERG: If patients have comorbidities that suggest they aren’t likely to
live for more than two or three years, it’s reasonable not to administer adjuvant
therapy.
I’ll give you two examples from my practice. The first is a 90-year-old
woman, who’s now 92, who received adjuvant FOLFOX.
Even though she walked with a walker because of arthritis, she was going to
the pool for water aerobics every day. She went to the senior citizens’ center
every day, and she was active within the limitations of her arthritis.
The other patient was in her mideighties, and we made a decision not to treat
her. Now I’m using FOLFOX to treat her for advanced disease and wishing
that I had treated her in the adjuvant setting because she had a high risk for
cancer recurrence.
Maybe her cancer would have recurred anyway, but we’re at the same point,
using the same drugs. She’s two years older, and I’m using it with palliative
intent rather than curative intent.
Track 3
 DR LOVE:
DR LOVE: Where are we in terms of clinical trials in the adjuvant setting?
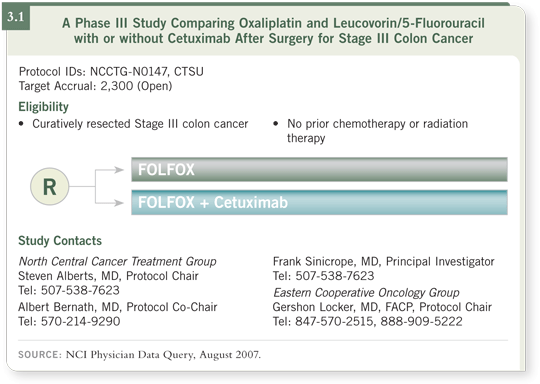
 DR GOLDBERG: The one trial open in the US is the NCCTG-N0147 study,
which is evaluating FOLFOX with or without cetuximab (3.1). It’s approximately
a third of the way to its final accrual, so it’s making progress. I believe
this is an important question to answer.
DR GOLDBERG: The one trial open in the US is the NCCTG-N0147 study,
which is evaluating FOLFOX with or without cetuximab (3.1). It’s approximately
a third of the way to its final accrual, so it’s making progress. I believe
this is an important question to answer.
Beyond that, it isn’t clear to me what the next chemotherapy/biologic question
ought to be. Some discussion took place about five drugs versus four —
FOLFOX and bevacizumab with or without cetuximab.
My experience with patients I’ve enrolled in CALGB-80405 in the metastatic
setting is that the ones who are randomly assigned to five drugs have a hard
time receiving treatment until disease progression. Most of them have shown
responses, but most of them have “said ‘uncle’” before they’ve received the
maximum potential benefit.
Track 6
 DR LOVE:
DR LOVE: What is your decision-making process regarding first-line
therapy in the metastatic setting?
 DR GOLDBERG: I initially attempt to enroll patients on the CALGB-80405
trial, which is a study of dealer’s choice of chemotherapy — FOLFOX or
FOLFIRI — combined with either cetuximab, bevacizumab or both.
DR GOLDBERG: I initially attempt to enroll patients on the CALGB-80405
trial, which is a study of dealer’s choice of chemotherapy — FOLFOX or
FOLFIRI — combined with either cetuximab, bevacizumab or both.
If a patient is not interested, I’ll tell him or her that FOLFOX and FOLFIRI
provide basically equivalent outcomes and that, in general, we’re adding
bevacizumab to first-line therapy.
I don’t believe that the NO16966 trial data presented at ASCO will change my
first-line approach off study. I will still offer people FOLFOX with bevacizumab
as first-line therapy.
The data from the NO16966 trial, which evaluated CAPOX versus FOLFOX
with or without bevacizumab, indicated that no difference in response rate
appeared when they added bevacizumab (Saltz 2007). Modest differences in
survival of about a month were evident.
Does that mean bevacizumab is not worth adding? Not to me. I believe it’s
worth adding bevacizumab to FOLFOX, but I have my eye on it. I’m watching
for additional information to either reinforce or change my opinion.
Select Publications

